Abstract
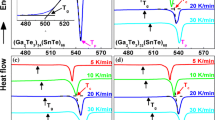
The crystallization kinetics of the chalcogenide glass Se0.8Te0.2 was studied by means of differential scanning calorimetry. The variation in partial area (X) with temperature (T) revealed that the transition from the amorphous to the crystalline phase occurs in two dimensions.
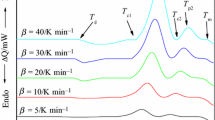
Activation energies were determined for both the glass transition (E t) and the crystallization (E c).E t was calculated from the variation inT g with the heating rate (a).E c was determined by three different methods: (i) variation inX withT, (ii) variation inT p witha, and (iii) variation inT c witha.E t andE c have values of 161.01±2.75 and 84.75 ±8.21 kJ/mol, respectively.
Zusammenfassung
Mittels DSC wurde die Kristallisierungskinetik des Chalkogenidglases Se0.8Te0.2 untersucht. Eine Änderung partieller Gebiete (X) mit der Temperatur (T) zeigte, daß der Übergang von der amorphen zur kristallinen Phase zweidimensional verläuft.
Es wurde die Aktivierungsenergie sowohl für den Glasübergang (E t) als auch für die Kristallisierung (E c) bestimmt.E t wurde mittels der Abhängigkeit vonT g von der Aufheizgeschwindigkeit (a) ermittelt.E c wurde auf drei verschiedene Wege bestimmt: (i) Änderung vonX in Abhängigkeit vonT, (ii) Änderung vonT p in Abhängigkeit vona und (iii) Änderung vonT c in Abhängigkeit vona. Die Werte vonE t undE c betragen 161.01±2.75 bzw. 84.75±8.21 kJ/mol.
Similar content being viewed by others
References
A. K. Agnihotri, A. Kumar and A. N. Nigam, J. Non-Ciyst. Solids, 101 (1988) 127.
L. Cheung, G. M. T. Foley, D. Fornia and B. E. Springett, Photogr. Sci. Eng. (USA), 26 (1982) 251.
V. Damodara Das and P. J. Lakshmi, Phys. Rev. B., 37 (1988) 720.
J. S. Vermak and J. Petruzzello, J. Appl. Phys., 53 (1982) 6809.
V. Damodara Das and P. J. Lakshmi, J. Appl. Phys., 62 (1987) 2376.
G. Pfister, J. Elect. Mat. 8 (1979) 789.
H. Yang, W. Wang and S. Min, J. Non-Cryst. Solids, 80 (1986) 503.
S. Mahadevan, A. Giridhar and A. K. Singh, J. Non-Cryst. Solids, 88 (1986) 11.
H. E. Kissinger, Anal. Chem., 29 (1957) 1702.
N. Rysavá, T. Spasev and L. Tichy, J. Thermal Anal., 32 (1987) 1015.
H. Yinnon and D. R. Uhlman, J. Non-Cryst. Solids, 54 (1983) 253.
H. E. Kissinger, J. Res. Not. Bur. Stand. 57 (1956) 217.
H. S. Chen, J. Non-Cryst. Solids, 27 (1978) 257.
J. E. Shelby, J. Non-Cryst. Solids, 34 (1979) 111.
J. Colemenero and J. M. Barandiaran, J. Non-Cryst. Solids, 30 (1978) 263.
J. A. Macmillan, J. Phys. Chem., 42 (1965) 3497.
B. G. Bagley and E. M. Vogel, J. Non-Cryst. Solids, 18 (1975) 29.
M. G. Scott, J. Mat. Sci., 13 (1978) 291.
V. R. V. Raman and G. E. Fish, J. Appl. Phys., 53 (1982) 2273.
D. W. Henderson, J. Non-Cryst. Solids, 30 (1970) 301.
K. Matusita, T. Konatsu and R. Yokota, J. Mat. Sci., 19 (1984) 291.
K. Matusita and S. Sakka, Phys. Chem. Glasses, 20 (1979) 81.
D. D. Thornburg and R. I. Johnson, J. Non-Cryst. Solids, 17 (1975) 2.
H. Yinnon and D. R. Uhlman, J. Non-Cryst. Solids, 54 (1983) 253.
R Frahn, J. Non-Cryst. Solids, 56 (1983) 255.
M. Cadergren and G. Backstrom, J. Non-Cryst. Solids, 37 (1980) 213.
D. R. Macfarlane, M. Matecki and M. Poulain, J. Non-Cryst. Solids, 64 (1984) 351.
K. Matusita and S. Sakka, J. Non-Cryst. Solids, 38–39 (1980) 741.
K. Matusita and S. Sakka, Bull. Inst. Chem. Res. Kyoto Univ., 59 (1981) 159.
A. Morotta, S. Saiello and A. Buri, J. Non-Cryst. Solids, 57 (1983) 473.
K. Matusita and S. Sakka, Thermochim. Acta, 33 (1979) 351.
S. H. Kandil, M. E. Kassem, M. A. El-Gamal and E. F. El-Wahidy, J. Mater. Sci. Let., 5 (1986) 112.
Author information
Authors and Affiliations
Additional information
This work was partly supported by a Grant-in-Aid for Scientific Research from the GTZ GmbH and DAAD, W. Germany.
Rights and permissions
About this article
Cite this article
Hafiz, M.M., Afify, N., Osman, M.A. et al. Crystallization kinetics of chalcogenide glass Se0.8 Te0.2 . Journal of Thermal Analysis 36, 2417–2426 (1990). https://doi.org/10.1007/BF01913639
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01913639