Abstract
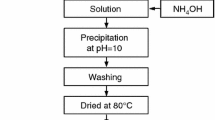
Aluminium hydroxide was prepared by precipitation from aluminium nitrate solution with ammonia solution. Thermal decomposition of the solid hydroxide was studied by means of TG, DTG and DTA.
The sample was thermally treated in the temperature interval between 200 °C and 1000 °C. X-ray phase analysis was used to study the phase compositions of the resulting products, and their surface areas were compared.
Zusammenfassung
Aus einer Aluminiumnitratlösung wurde mit Ammoniaklösung durch Präzipitation Aluminiumhydroxid gefertigt. Mittels TG, DTA und DTG wurde die thermische Zersetzung des festen Hydroxides untersucht. Die Probe wurde Temperaturen zwischen 200 °C und 1000 °C ausgesetzt. Die Phasenzusammensetzung der erhaltenen Produkte wurde mittels Röntgenphasenanalyse untersucht und die Oberflächenstrukturen verglichen.
Резюме
Методами ТГ, ДТГ и ДТА и зучено термическое разложение осажденн ой гидроокиси алюминия, полученной из растворов нитрата алюминия и аммиака. Образец терм ически обрабатывался в темп ературном интервале 200–1000°. Фазовый состав образ ующихся при этом продуктов был изучен с помощью рентгенофа зового анализа и сопоставле на их удельная площадь поверхности.
Similar content being viewed by others
References
B. C. Lippens and J. J. Steggerda, in B. G. Linsen (Ed.); Physical and Chemical Aspects of Adsorbents and Catalysts, Academic Press, London and New York 1970, Ch. 4.
B. C. Gates, J. R. Katzer and G. C. A. Schuit, Chemistry of Catalytic Processes, McGraw-Hill, New York 1979.
V. A. Ushakov and E. M. Moroz, Kin. and Catal., 26 (1985) 832.
H. Knozinger and P. Ratnasamy, Catal. Rev.-Sci.-Eng., 17(1978) 31.
H. C. Stumpf, A. S. Russel, J. W. Newsome and C. M. Tucker, Ind. Eng. Chem., 42 (1950) 1398.
D. L. Cocke, E. D. Johnson and R. P. Merrill, Catal. Rev.-Sci. Eng., 26 (1984) 163.
J. R. Anderson, Structure of metallic catalysts, Academic Press, 1975, Ch. 2.
H. Pines and W. O. Haag, J. Amer. Chem. Soc., 82 (1960) 2471.
R. A. Shkrabina, E. M. Moroz and E. A. Levitskii, Kin. and Catal., 22 (1981) 1015.
M. Jayamani and C. N. Pillai, J. Catal., 82 (1983) 485.
H. P. Rooksby, in G. Brown (Ed.), The X-ay identification and crystal structure of clay minerals, 1972, p. 354.
R. C. Mackenzie, Scifax Differential Thermal Analysis Data Index, Cleaver-Hume, London 1962.
G. A. El-Shobaky, N. M. Ghoneim and E. A. Sultan, Thermochim. Acta, 63 (1983) 39.
D. S. Maciver, H. H. Tobin and R. T. Barth, J. Catal., 2 (1963) 485.
A. S. Russell and C. N. Cochran, Ind. Eng. Chem., 42 (1950) 1332.
S. Janiak and J. Wrzyszcz, “Preparation of Catalyst 1”, Elsevier, Amsterdam 1976, p. 663.
N. W. Al-Derzi and F. A-Mashta, Paper sent for publication.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Al-Mashta, F., Al-Derzi, N., Al-Saadi, A. et al. Preparation of aluminas. Journal of Thermal Analysis 34, 269–277 (1988). https://doi.org/10.1007/BF01913393
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01913393