Abstract
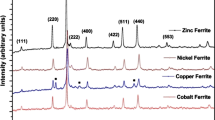
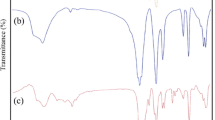
Fine particle cobalt dopedγ-Fe2O3 and Mn-Zn ferrites have been prepared by the thermal decomposition of N2H5Co x Fe1−x (N2H3COO)3.H2O wherex=1–10 atom% and (N2H5)3MnxZn1−x Fe2(N2H3COO)9· 3H2O wherex=0.2–0.8, respectively. Formation of these oxide materials has been confirmed by thermogravimetry and X-ray powder diffraction patterns. The fine particle nature of these oxide materials is evident from particle size analysis and surface area measurements.
Zusammenfassung
Mit fein verteiltem Kobalt versetztesγ-Fe2O3 sowie Mn-Zn Ferrite wurden durch thermische Zersetzung von N2H5Co x Fe1−x (N2H3COO)3·H2O mit jc=1–10 Atomprozent bzw. (N2H5)3Mn x Zn1−x Fe2(N2H3COO)9·3H2O mitx=0,2–0,8 hergestellt. Die Bildung dieser Oxidstoffe wurde durch Thermogravimetrie und Pulverdiffraktionsmethoden bekräftigt. Die Feinkornstruktur dieser Oxidstoffe wird durch Korngrößenverteilungs- und Oberflächenmessungen augenscheinlich.
Резюме
Термическим разложе нием соединений N2H5Co x Fe1−x (N2H3COO)3·H2O, гдеx-1–10 атомных%, и (N2H5)3Mn x Zn1−x Fe2(N2H3COO)9 · 3H2O, гдеx=0,2–0,8, были получены, соответственно, леги рованный кобальтом порошкообразныйγ-Fe2O3 и Mn-Zn ферриты. Образование таких ок сидных материалов бы ло подтверждено термог равиметрией и порошковым рентгено структурным анализо м. Тонкопорошковая при рода этих оксидных материалов доказана ситовым анализом и из мерением площади поверхности.
Similar content being viewed by others
References
T. A. Arnoldussen and E. M. Ross, Annual Review of Material Science, 15 (1985) 705.
G. I. Kolendnko and V. V. Chaplinski, Izu. Leningr. Electrotekh. Inst., 167 (1975) 121.
A. K. Nikumba, K. S. Rane and A. J. Mukhadkhor, J. Mat. Sci., 18 (1983) 3415.
B. K. Das, Preparation and Characterisation of Materials, Ed. J. M. Honig and C. N. R. Rao, Academic Press, New York 1981, p. 75.
P. Ravindranathan and K. C. Patil, J. Mat. Sci. Letter, 5 (1986) 22.
P. Ravindranathan and K. C. Patil, Amer. Ceram. Soc. Bull., 66 (1987) 688.
T. T. Srinivasan, P. Ravindranathan, L. E. Cross, R. Roy, R. Newham, S. G. Shankar and K. C. Patil, J. Appl. Phys., 63 (1988) 5789.
P. Ravindranathan and K. C. Patil, Proc. Indian Acad. Sci. (Chem. Sci)., 95 (1985) 345.
Powder Diffraction File Inorganic Vol PD25-5IRB Joint committee of diffraction standards, Pennsylvania, 1960.
G. W. Van Oosterhout, Acta. Crystallogr., 13 (1960) 932.
Ju. Ja. Konakhovich and Ju. G. Saksonov, Krystallografija, SSSR, 8 (1963) 18 (in Russian).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Suresh, K., Mahesh, G.V. & Patil, K.C. Preparation of cobalt doped γ-Fe2O3 and Mn-Zn ferrites by the thermal decomposition of the hydrazine precursors. Journal of Thermal Analysis 35, 1137–1143 (1989). https://doi.org/10.1007/BF01913031
Issue Date:
DOI: https://doi.org/10.1007/BF01913031