Abstract
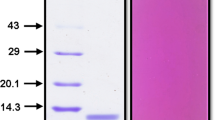
TaTI (Torresea acreana trypsin inhibitor), a new member of the Bowman-Birk trypsin inhibitor family, was purified from seeds ofTorresea acreana, one of the two known species ofTorresea, a Brazilian native Leguminosae of the Papilionoideae subfamily. Purification was performed by acetone fractionation, anion-exchange chromatography, and gel filtration. The TaTI appears asM r 7000 in SDS-PAGE under reducing conditions. There are 63 amino acid residues present in the TaTI sequence, which was confirmed by mass spectrometry (8388 daltons). The putative reactive sites residues were Lys-15 and Arg-42 at the first and second site, respectively. The antibodies raised against TcTI2,Torresea cearensis trypsin inhibitor 2, showed a cross-reaction with TaTI, but not with other Bowman-Birk inhibitors purified from Leguminosae. The inhibition constants of TaTI and TcTI2 were comparable when measured against trypsin, chymotrypsin, and factor XIIa, but not on plasmin. The latter was tenfold more effectively inhibited by TcTI2 then by TaTI. Neither TaTI nor TcTI2 affects thrombin, plasma kallikrein, or factor Xa.
Similar content being viewed by others
References
Chase, T., and Shaw, E. (1970). Titration of trypsin, plasmin and thrombin withp-nitrophenyl-p-guanidinobenzoate HCl,Meth. Enzymol. 19, 20–27.
Erlanger, B. F., Kokowsky, N., and Cohen, E. (1961). Preparation and properties of two new chromogenic substrates of trypsin,Arch. Biochem. Biophys. 95, 271–278.
Green, T. R., and Ryan, C. (1972). Wound-induced proteinase inhibitor in plant leaves. A possible defense mechanism against insects,Science 175, 776–777.
Hopp, T. P., and Woods, K. R. (1981). Prediction of protein determinants from amino acid sequences,Proc. Natl. Acad. Sci. USA 78, 3824–3828.
Kennedy, A. R. (1993a).In vitro studies of anticarcinogenic protease inhibitors, inProtease Inhibitors as Cancer Chemopreventive Agents (Troll, W., and Kennedy, A. R., eds.), Plenum Press, New York, pp. 65–91.
Kennedy, A. R. (1993b). Overview of anticarcinogenic activity of protease inhibitors, inProtease Inhibitors as Cancer Chemopreventive Agents (Troll, W., and Kennedy, A. R., eds.), Plenum Press, New York, pp. 9–64.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4,Nature 227, 680–685.
Laskowski, M., Jr., and Kato, I. (1980). Protein inhibitors of proteinases,Annu. Rev. Biochem. 49, 593–626.
Lin, G., Bode, W., Huber, R., Chi, C., and Engh, R. A. (1993). The 0.25-nm X-ray structure of the Bowman-Birk-type inhibitor from mung bean in ternary complex with porcine trypsin,Eur. J. Biochem. 212, 549–555.
Neurath, H. (1984). Evolution of proteolytic enzymes,Science 224, 350–357.
Nilsson, B. O., and Larsson, A. (1990). Intrasplenic immunization with minute amounts of antigen,Immunol. Today 11, 10–12.
Odani, S., and Ikenaka, T. (1972). Studies on soybean trypsin inhibitors. IV. Complete amino acid sequence and the anti-proteinase sites of Bowman-Birk soybean proteinase inhibitor,J. Biochem. 71, 839–848.
Odani, S., Koide, T., and Ono, T. (1986). Wheat germ trypsin inhibitors. Isolation and structural characterization of single-headed and double-headed inhibitors of the Bowman-Birk type,J. Biochem. 100, 975–983.
Oliva, M. L. V., Grisolia, D., Sampaio, M. U., and Sampaio, C. A. M. (1982). Properties of highly purified human plasma kallikrein,Agents Actions 9, 52–57.
Richardson, M. (1987). The proteinase inhibitors of plants and microorganisms,Phytochemistry 16, 159–169.
Richardson, M. (1991). Seed storage proteins. The enzyme inhibitors,Meth. Plant Biochem. 5, 259–305.
Sampaio, C. A. M., Wong, S. C., and Shaw, E. (1974). Human kallikrein. Purification and preliminary characterization,Arch. Biochem. Biophys. 165, 133–139.
Sampaio, M. U., Tanaka, A. T., Oliva, M. L. V., Batista, I. F. C., Motta, G., Stella, R. C. R., and Sampaio, C. A. M. (1992). Plant proteinase inhibitor. Action on blood clotting contact phase enzymes, inProceedings from I CONBRAP, pp. 57–65.
Suzuki, A., Tsunogae, Y., Tanaka, I., Yamane, T., Ashida, T., Norioka, S., Hara, S., and Ikenaka, T. (1987). The structure of Bowman-Birk type protease inhibitor A-II from peanut (Arachis hypogaea) at 3.3 A resolution,J. Biochem. 101, 267–274.
Tanaka, A. S., Sampaio, M. U., Marangoni, S., Oliveira, B., Novello, J. C., Oliva, M. L. V., Fink, E., Fritz, H., and Sampaio, C. A. M. (1996). Purification and primary structure determination of a Bowman-Birk trypsin inhibitor fromTorresea cearensis seeds, In preparation.
Tsunogae, Y., Tanaka, I., Yamane, T., Kikkawa, J., Ashida, T., Ishikawa, C., Watanabe, K., Nakamura, S., and Takahashi, K. (1986). Structure of the trypsin-binding domain of Bowman-Birk type inhibitor and its interation with trypsin,J. Biochem. 100, 1637–1646.
Voller, A. (1980). Heterogeneous enzyme-immunoassays and their applications, inEnzyme-Immunoassay (Maggio, E. T., ed.), CRC Press, Boca Raton, Florida, pp. 181–196.
Wilson, K. A., and Chen, J. C. (1983). Amino acid sequence of mung bean trypsin inhibitor and its modified forms appearing during germination,Plant Physiol. 71, 341–349.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, A.S., Sampaio, M.U., Mentele, R. et al. Sequence of a new Bowman-Birk inhibitor fromTorresea acreana seeds and comparison withTorresea cearensis trypsin inhibitor (TcTI2). J Protein Chem 15, 553–560 (1996). https://doi.org/10.1007/BF01908537
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01908537