Summary
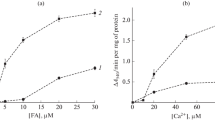
Guinea pig hearts were subjected to low-flow perfusion (0.3 ml/g fresh weight/min) with an oxygen depleted perfusate. Fatty acids (palmitic or oleic acid), added to the perfusate, accelerated in a dose-dependent manner the anoxic decay of creatine phosphate and ATP, impaired lactate production and augmented enzyme release (lactate dehydrogenase, malate dehydrogenase). Palmitic and olcic acid, however, differed distinctly in their deleterious effect, this being greater for oleic acid. After 60 min anoxic low-flow perfusion with 11 mM glucose and 0.2 mM of either fatty acid, complexed in 5∶1 molar relationship to albumin, the creatine phosphate content with palmitate is 39% greater than with oleate, the ATP content 23%, lactate production 15% greater, and release of malate dehydrogenase 24% lower, but the elevated contents of long-chain acyl CoA and acyl carnitine are not significantly different for the two fatty acids. These results accord with earlier experiences on subcellular systems showing that the physicochemical effects of the oleyl residue are more harmful than those of the palmityl residue.
Similar content being viewed by others
References
Adams RJ, Cohen DW, Gupte S, Johnson JD, Wallick ET, Wang T, Schwartz A (1979) In vitro effects of palmitoyl carnitine on cardiac plasma membrane Na, K-ATPase, and sarcoplasmic reticulum Ca2+-ATPase and Ca2+ transport. J Biol Chem 254:12404–12410
Bergmeyer HU (1974) Methods of Enzymatic Analysis. Academic Press, New York
Challoner DR, Steinberg D (1966) Oxidative metabolism of myocardium as influenced by fatty acids and epinephrine. Am J Physiol 211:897–902
Corr PB, Gross RW, Sobel BE (1984) Amphiphatic metabolites and membrane dysfunction in ischemic myocardium. Circ Res 55:135–154
DeLeiris J, Opie LH (1978) Effect of substrates and of coronary artery ligation on mechanical performance and on release of lactate dehydrogenase and creatine phosphokinase in isolated working rat hearts. Cardiovasc Res 12:585–596
DeLeiris J, Opie LH, Lubbe WF (1975) Effects of free fatty acid and glucose on enzyme release in experimental myocardial infarction. Nature 253:746–747
Finch SAE, Piper HM, Spieckermann PG, Stier A (1985) Anoxia influences the lateral diffusion of a lipid probe in the plasma membrane of isolated cardiac myocytes. Basic Res Cardiol 80 (suppl 1):149–152
Glatz JF, Baerwaldt CCF, Veerkamp JH, Kempen HJM (1984) Diurnal variation of cytosolic fatty acid-binding protein content and of palmitate oxidation in rat liver and heart. J Biol Chem 259:4295–4300
Glatz JFC, Paulussen RJA, Veerkamp JH (1985) Fatty acid binding proteins from heart. Chem Phys Lipids 38:115–129
Idell-Wenger JA, Grotjohann LW, Neely JR (1978) Coenzyme A and carnitine distribution in normal and ischemic hearts. J Biol Chem 253:4310–4318
Kammermeier H, Wein B, Gerards P, Lang U, Wendtland B, Schmitz D, Rose H (1985) Barriers in cardiac substrate supply. Basic Res Cardiol 80 (suppl 2):89–92
Katz AM, Messineo FC (1981) Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ Res 48:1–16
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Amer Statist Ass 47:583–621
Mjøs OD, Kjekshus JK, Lekven J (1974) Importance of free fatty acids as a determinant of myocardial oxygen consumption and myocardial ischemic injury during norepinephrine infusion in dogs. J Clin Invest 53:1290–1299
Morel F, Lauquin G, Lunardi J, Duszynski J, Vignais PV (1974) An appraisal of the functional significance of an inhibitory effect of long chain acyl-CoA on mitochondrial transports. FEBS Lett 39:133–138
Ockner RK, Manning JA (1974) Fatty acid-binding protein in small intestine. Identification, isolation and evidence for its role in cellular fatty acid transport. J Clin Invest 54:326–338
Owens K, Kennet FF, Weglicki WB (1982) Effects of fatty acid intermediates on Na+−K+-ATPase activity of cardiac sarcolemma. Am J Physiol 242:H456-H461
Pande SV, Blanchaer MC (1971) Reversible inhibition of mitochondrial adenosine diphosphate phosphorlyation by long chain acyl coenzyme A esters. J Biol Chem 246:402–411
Pearce FJ, Forster J, De Leeuw G, Williamson JR, Tutwiler GF (1979) Inhibition of fatty acid oxidation in normal and hypoxic perfused rat hearts by 2-tetradecylglycidic acid. J Mol Cell Cardiol 12:893–915
Piper HM, Schwartz P, Spahr R, Hütter JF, Spieckermann PG (1984) Early enzyme release from myocardial cells is not due to irreversible cell damage. J Mol Cell Cardiol 16:385–388
Piper HM, Sezer O, Schwartz P, Hütter JF, Schweickhardt C, Spieckermann PG (1984) Acylcarnitine effects on isolated cardiac mitochondria and erythrocytes. Basic Res Cardiol 79:186–198
Piper HM, Sezer O, Schwartz P, Hütter JF, Spieckermann PG (1983) Fatty acid-membrane interactions in isolated cardiac mitochondria and erythrocytes. Biochim Biophys Acta 752:193–203
Prinzen FW, Van der Vusse GJ, Arts T, Roemen THM, Coumans WA, Reneman RS (1984) Accumulation of nonesterified fatty acids in ischemic canine myocardium. Am J Physiol 247:H264-H272
Rovetto MJ, Lamberton WF, Neely JR (1975) Mechanisms of glycolytic inhibition in ischemic rat hearts. Circ Res 37:742–751
Spector AA, Brennan DE (1972) Effect of free fatty acid structure on binding to rat liver mitochondria. Biochim Biophys Acta 260:433–438
Spector AA, Fletcher JE (1978) Transport of fatty acids in circulation. In: Dietschy JM, Gotto jr AM, Ontko JA (eds) Disturbances in Lipid and Lipoprotein Metabolism. American Physiological Society, Bethesda, pp 229–249
Spector AA, Fletcher JE, Ashbrook JD (1971) Analysis of long-chain free fatty acid binding to bovine serum albumin by determination of stepwise equilibrium constants. Biochemistry 10:3229–3232
Van der Vusse GJ, Reneman RS (1984) The myocardial non-esterified fatty acid controversy. J Mol Cell Cardiol 16:677–682
Van der Vusse GJ, Roemen THM, Flameng W, Reneman R (1983) Serum-myocardial gradients of non-esterified fatty acids. Biochim Biophys Acta 752:361–370
Vik-Mo H, Mjøs OD (1981) Influence of free fatty acids on myocardial oxygen consumption and ischemic injury. Am J Cardiol 48:361–365
Wojtczak L (1976) Effect of long-chain fatty acids and acyl-CoA on mitochondrial permeability, transport and energy-coupling processes. J Bioenerg Biomembr 8:293–311
Wosilait WD, Soler-Argilaga C, Nagy P (1976) A theoretical analysis of the binding of palmitate by human serum albumin. Biochem Biophys Res Commun 71:419–426
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Piper, H.M., Das, A. The role of fatty acids in ischemic tissue injury: difference between oleic and palmitic acid. Basic Res Cardiol 81, 373–383 (1986). https://doi.org/10.1007/BF01907458
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01907458