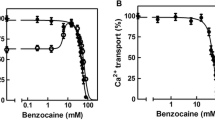
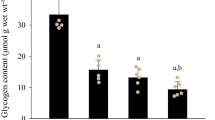
Summary
Sarcolemmal membranes were prepared from slow-twitch (red) and fasttwitch (white) skeletal muscle of the rat. A sensitive adenylate cyclase assay was used and basal, fluoride- and catecholamine-stimulated activities measured. The greaterin vivo sensitivity of red muscle to the effects of catecholamines correlates, in the present study, with approximately a twofold stimulation of its sarcolemmal adenylate cyclase with isoproterenol (10 μm). The white muscle enzyme, on the other hand, is only minimally stimulated (20%) at the same concentration of β-adrenergic agonist. Fast-twitch muscle is known to be physiologically insensitive to catecholaminein vivo.
A course of sciatic nerve denervation was followed to further distinguish these two metabolic types of skeletal muscle and their respective adenylate cyclases. The slow-twitch muscle enzyme activities were completely and permanently lost on denervation. The white muscle enzyme, however, recovered almost completely after an initial reduction in specific activity the first week. Interestingly, the NaF-stimulated activity lagged behind both the basal and hormone-stimulated activities of the white muscle enzyme, in returning to control levels. The activities of cyclic nucleotide phosphodiesterase were evaluated in homogenates of the two muscle types in innervated rats and following denervation, in order to further define the neural influence on skeletal muscle cyclic nucleotide metabolism.
The results suggest that the motor nerve may regulate some of the metabolic properties of slow-twitch muscle (which may involve cyclic AMP) by controlling the responsiveness of its sarcolemmal-bound adenylate cyclase system.
Similar content being viewed by others
References
Albuquerque, E.X., Schuh, R.T., Kaufman, F.C. 1971. Early membrane depolarization of the fast mammalian muscle after denervation.Pfluegers Arch. Gesamte Physiol. 328:36
Albuquerque, E.X., Warnick, J.E., Tasse, J.R., Sansone, F.M. 1972. Effects of vinblastine and colchicine on neural regulation of fast and slow skeletal muscles of the rat.Explor. Neurol. 37:607
Aurbach, G.D., Fedak, S.A., Woodard, C.J., Palmer, J.S., Hauser, D., Troxler, F. 1974. β-Adrenergic receptor: Stereospecific interaction of iodinated β-blocking agent with high affinity site.Science 186:1223
Axelsson, J., Thesleff, S. 1959. A study of supersensitivity in denervated mammalian skeletal muscle.J. physiol. (London) 149:178
Bowman, W.C., Nott, M.W. 1969. Actions of sympathomimetic amines and their antagonists on skeletal muscle.Pharmacol. Rev. 21:27
Bowman, W.C., Raper, C. 1962. Adrenaline and slow-contracting skeletal muscle.Nature (London) 193:41
Bowman, W.C., Raper, C. 1965. The effects of sympathomimetic amines on chronically denervated skeletal muscle.Br. J. Pharmacol. Chemother. 24:98
Canal, N., Frattola, L., Smirne, S. 1975. The metabolism of cyclic 3′-5′-adenosine monophosphate (cAMP) in diseased muscle.J. Neurol. 208:259
Carlsen, R.C. 1975. The possible role of cyclic AMP in the neurotrophic control of skeletal muscle.J. Physiol. (London) 247:343
Close, R.I. 1972. Dynamic properties of mammalian skeletal muscles.Physiol. Rev. 52:129
Drachman, D.B. (Ed.) 1974. Trophic functions of the neuron.Ann. N.Y. Acad. Sci. 228:1
Festoff, B.W., Engel, W.K. 1974.In vitro analysis of the general properties and junctional receptor characteristics of skeletal muscle membranes. Isolation, purification, and partial characterization of sarcolemmal fragments.Proc. Nat. Acad. Sci. USA 71:2435
Festoff, B.W., Oliver, K.L., Reddy, N.B. 1977.In vitro studies of skeletal muscle membranes. Effects of denervation on the macromolecular components of cation transport in red and white skeletal muscle.J. Membrane Biol. 32:345
Goffart, M., Ritchie, J.M. 1952. The effect of adrenaline on the contraction of mammalian skeletal muscle.J. Physiol. (London) 116:357
Guth, L. 1968. “Trophic” influences of nerve on muscle.Physiol. Rev. 48:645
Guth, L. 1974. “Trophic” functions.In: The Peripheral Nervous System. J.I. Hubbard, editor. pp. 181–194. Plenum Press, New York
Gutmann, E., Zelena, J. 1962. Morphological changes in the denervated muscle.In: The Denervated Muscle. E. Gutmann, editor. pp. 57–102. Publishing House of the Czechoslovak Academy of Science. Prague
Janaki, S., Susheela, A.K. 1966. Enzyme histochemistry of muscle with special reference to the Duchenne type of muscular dystrophy.Neurology (Bombay) 14:174
Jones, R., Vrbova, G. 1974. Two factors responsible for the development of denervation hypersensitivity.J. Physiol. (London) 236:517
Lefkowitz, R.J. 1973. Isolated beta-adrenergic binding sites: A potential assay vehicle for catecholamines.Pharmacol. Rev. 25:259
Lentz, T.L. 1972. A role of cyclic AMP in a neurotrophic process.Nature, New Biol. 238:154
Lilienthal, J.L., Jr., Zierler, K.L., Folk, B.P., Buka, R., Riley, M.J. 1950. A base and system for analysis of muscle constituents.J. Biol. Chem. 182:501
Lomo, T., Rosenthal, J. 1972. Control of ACh sensitivity by muscle activity in the rat.J. Physiol. (London) 221:493
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. 1951. Protein measurement with the folin phenol reagent.,J. Biol. Chem. 193:265
Mawatari, S., Takagi, A., Rowland, L.P. 1974. Adenyl cyclase in normal and pathologic human muscle.Arch. Neurol. 30:96
McMahon, D. 1974. Chemical messengers in development: A hypothesis.Science 185:1012
Musick, J., Hubbard, J.I. 1972. Release of protein from mouse motor nerve terminals.Nature (London) 237:279
Oh, T.H. 1975. Neurotrophic effects: Characterization of the nerve extract that stimulates muscle development in culture.Explor. Neurol. 46:432
Reddy, N.B., Engel, W.K., Festoff, B.W. 1976a.In vitro studies of skeletal muscle membranes. Characterization of a phosphorylated intermediate of sarcolemmal (Na++K+) ATPase.Biochim. Biophys. Acta 433:365
Reddy, N.B., Oliver, K.L., Engel, W.K., Festoff, B.W. 1976b. Neurotrophic control of adenylate cyclase in fast and slow twitch skeletal muscle.Fed. Proc. 35:378
Redfern, P., Thesleff, S. 1971. Action potential generation in denervated rat skeletal muscle.Acta Physiol. Scand. 81:557
Salafsky, B., Bell, J., Prewitt, M.A. 1968. Development of fibrillation potentials in denervated fast and slow skeletal muscle.Am. J. Physiol. 215:637
Salomon, Y., Londos, C., Rodbell, M. 1974. A highly sensitive adenylate cyclase assay.Analyt. Biochem. 58:541
Silinsky, E.M., Hubbard, J.I. 1973. Release of ATP from rat motor nerve terminals.Nature (London) 243:404
Sushela, A.K., Kaul, R.D., Sachdeva, K., Singh, N. 1975. Adenyl cyclase activity in Duchenne dystrophic muscle.J. Neurol. Sci. 24:361
Sutherland, E.W., Robison, G.A. 1966. The role of cyclic 3′-5′-AMP in response to catecholamines and other hormones.Pharmacol. Rev. 18:145
Thompson, W.J., Appleman, M.M. 1971. Characterization of cyclic nucleotide phosphodiesterases of rat tissues.J. Biol. Chem. 246:3145
Thompson, W.J., Brooker, G., Appleman, M.M. 1974. Assay of cyclic nucleotide phosphodiesterases with radioactive substrates.Meth. Enzymol. 38:205
Tower, S.S. 1935. Atrophy and degeneration in skeletal muscle.Am. J. Anat. 56:1
Zak, R. 1962. Proteins in the denervated muscle. Change in their quantity, properties and metabolism.In: The Denervated Muscle. E. Gutmann, editor. p. 273. Publishing House of the Czechoslovak Academy of Science, Prague
Zalin, R.J., Montague, W. 1974. Changes in adenylate cyclase, cyclic AMP, and protein kinase levels in chick myoblasts, and their relationship to differentiation.Cell 2:103.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Festoff, B.W., Oliver, K.L. & Reddy, N.B. In Vitro studies of skeletal muscle membranes. J. Membrain Biol. 32, 331–343 (1977). https://doi.org/10.1007/BF01905226
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01905226