Abstract
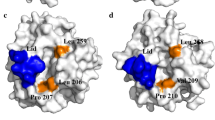
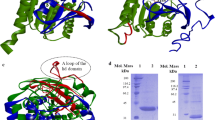
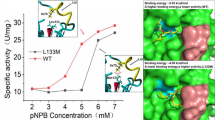
To reveal the functional role of Glu87 and Trp89 in the lid ofHumicola lanuginosa lipase, site-directed mutagenesis at Glu87 and Trp89 was carried out. The catalytic performance of wild-type and mutated lipases was studied in transesterification reactions in cyclohexane at a controlled water activity. Two different acyl donors were used in the investigation: tributyrin, a natural substrate for a lipase, and vinyl butyrate, an activated ester suitable for fast and efficient lipase-catalyzed transformations in preparative organic synthesis. As acyl acceptor 1-heptanol was used. The Glu87Ala mutation decreased theV max,app value with tributyrin and vinyl butyrate by a factor of 1.5 and 2, respectively. TheK m,app for tributyrin was not affected by the Glu87Ala mutation, but theK m,app for vinyl butyrate increased twofold compared to the wild-type lipase. Changing Trp89 into a Phe residue afforded an enzyme with a 2.7- and 2-fold decreasedV max,app with the substrates tributyrin and vinyl butyrate, respectively, compared to the wild-type lipase. No significant effects on theK m,app values for tributyrin or vinyl butyrate were seen as a result of the Trp89Phe mutation. However, the introduction of a Glu residue at position 89 in the lid increased theK m,app for tributyrin and vinyl butyrate by a factor of >5 and 2, respectively. The Trp89Glu mutated lipase could not be saturated with tributyrin within the experimental conditions (0–680 mM) studied here. With vinyl butyrate as a substrate theV max,app was only 6% of that obtained with wild-type enzyme.
Similar content being viewed by others
References
Beer, H. D., Wohlfahrt, G., McCarthy, J. E. G., Menge, U., Schomburg, D., and Schmid, R. D. (1993). Poster presentation at Lipases: Structure, function, and protein engineering, Elsinore, Denmark.
Boel, E., and Huge-Jensen, B. (1988).Eur. Pat. Appl. 305, 216.
Brzozowski, A. M., Derewenda, U., Derewenda, Z. S., Dodson, G., Lawson, D. M., Turkenburg, J. P., Björkling, F., Huge-Jensen, B., Patkar, S. A., and Thim, L. (1991).Nature 351, 491–494.
Derewenda, U., Brzozowski, A. M., Lawson, D. M., and Derewenda, Z. S. (1992).Biochemistry 31, 1532–1541.
Derewenda, U., Swenson, L., Green, R., Wei, Y., Yamaguchi, S., Joerger, R., Haas, M. J., and Derewenda, Z. S. (1994).Protein Eng. 7, 551–557.
Derewenda, Z. S. (1994).Adv. Protein Chem. 45, 1–52.
Euranto, E. K. (1969). InThe chemistry of Carboxylic Acids and Esters (Patai, S., ed.), Wiley, New York, pp. 505–589.
Fersht, A. (1985). InEnzyme Structure and Mechanism, 2nd ed., Freeman, New York.
Holmquist, M., Martinelle, M., Berglund, P., Clausen, I. G., Patkar, S., Svendsen, A., and Hult, K. (1993).J. Protein Chem. 12, 749–757.
Holmquist, M., Martinelle, M., Clausen, I. G., Patkar, S., Svendsen, A., and Hult, K. (1994).Lipids 29, 599–603.
Joerger, R. D., and Haas, M. J. (1994).Lipids 29, 377–384.
Kazlauskas, R. J. (1994).Tibtech. 12, 464–472.
Lawson, D. M., Brzozowski, A. M., Rety, S., Verma, C., and Dodson, G. G. (1994).Protein Eng. 7, 543–550.
Nelson, R. M., and Long, G. C. (1989).Anal. Biochem. 180, 147–151.
Norin, M., Haeffner, F., Achour, A., Norin, T., and Hult, K. (1994).Protein Sci. 3, 1493–1503.
Ollis, D. I., Cheah, E., Cygler, M., Dijkstra, B., Frolow, F., Franken, S. M., Harel, M., Remington, S. J., Silman, I., Schrag, J., Sussman, J., Verschueren, K. H. G., and Goldman, A. (1992).Proteins 5, 197–211.
Rubin, B. (1994).Nature Struct. Biol. 1, 568–572.
Yamaguchi, S., Mase, T., and Tekeuchi, K. (1992).Biosci. Biotechnol. Biochem,56, 315–319.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holmquist, M., Clausen, I.G., Patkar, S. et al. Probing a functional role of Glu87 and Trp89 in the lid ofHumicola lanuginosa lipase through transesterification reactions in organic solvent. J Protein Chem 14, 217–224 (1995). https://doi.org/10.1007/BF01886762
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01886762