Abstract
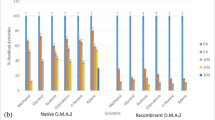
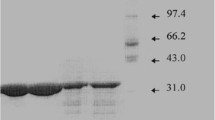
The alkaline proteases subtilisin Carlsberg and alcalase possess substantial enzymatic activity even when dissolved in ethanol. The crude enzymes were purified by gel filtration and the main fractions suspended in ethanol to give a translucent suspension. Both the supernatant and the resuspended precipitate after high-speed centrifugation were found to have enzymatic activities. The solubility of subtilisin Carlsberg in anhydrous ethanol was found to be 45.1μg/ml and that of alcalase was 48.1μg/ml by Coomassie blue dye-binding method using bovine serum albumin as a standard. In the presence of water, the solubility of both enzymes increased with water content. The stability of enzymes incubated in ethanol was assayed by their amidase and transesterase activities using Ala-Ala-Pro-Phe-pNA as substrate in phosphate buffer (pH8.2) and Moz-Leu-OBzl as substrate in anhydrous ethanol, respectively. The soluble enzymes have a half-life of about 36 hr and that of suspended enzymes about 50 hr in the amidase activity assay, whereas the same soluble enzymes have a half-life of about several hours and that of suspended enzymes 1 h by the transesterase activity assay. The stability of both enzymes decreased as water concentration increased. The diastereoselectivity of the enzyme-catalyzed hydrolysis of diastereo pairs of tetrapeptide esters,l-Ala-l-Ala-(d-orl-)Pro-l-Phe-OMe andl-Ala-l-Ala-(d-orl-)Ala-l-Phe-OMe, in phosphate is as high as that of the transesterification of these substrates in ethanol. It is concluded that active sites and selectivity of alkaline serine proteases in anhydrous alcohol are probably very similar to those in aqueous solution in spite of the fact that a lower reactivity is usually associated with the enzymes in nonaqueous solvents.
Similar content being viewed by others
References
Affleck, R., Xu, Z. F., Suzawa, V., Focht, K., Clark, D. S., and Dordick, J. S. (1992).Proc. Natl. Acad. Sci. USA 89, 1100–1104.
Arnold, F. H. (1988).Protein Eng. 2, 21–25.
Arnold, F. H. (1990).Trends Biotechnol. 8, 224–249.
Barbas, III, C. F., and Wong, C. H. (1987).J. Chem. Soc. Chem. Commun. 1987, 532–534.
Bott, R., Ultsch, M., Kossiakoff, A., Graycar, T., Katz, B., and Power, S. (1988).J. Biol. Chem. 263, 7895–7906.
Brink, L. E. S., Tramper, J., Luyben, K. Ch. A. M., and Riet, K. V. (1988).Enzyme Microb. Technol. 10, 736–743.
Chen, S.-T., Chen, S.-Y., Hsiao, S.-C., and Wang, K.-T. (1991a).Biotech. Lett. 13, 773–778.
Chen, S.-T., Hsiao, S.-C., and Wang, K.-T. (1991b).Bioorg. Med. Chem. Lett. 1, 445–450.
Chen, S.-T., Chen, S.-Y., and Wang, K.-T. (1992a).J. Org. Chem. 57, 6960–6965.
Chen, S.-T., Hsiao, S.-C., Chiou, A.-J., Wu, S.-H., and Wang, K.-T. (1992b).J. Chin. Chem. Soc. 39, 91–99.
Chen, S.-T., Chen, S.-Y., Tu, C.-C., and Wang, K.-T. (1993a).Bioorg. Med. Chem. Lett. 3, 1643–1648.
Chen, S.-T., Tu, C.-C., Chen, S.-Y., Huang, H.-C., and Wang, K.-T. (1993b).Bioorg. Med. Chem. 1, 361–367.
Dordick, J. S. (1992).Biotechnol. Prog. 8, 259–267.
Erp, S. H. M. V., Kamenskaya, E. O., and Khmelnitsky, Y. L. (1991).Eur. J. Biochem. 202, 379–384.
Fitzpatrick, P. A., and Klibanov, A. M. (1991).J. Am. Chem. Soc. 113, 3166–3171.
Fitzpatrick, P. A., Steinmetz, A. C. U., Ringe, D., and Klibanov, A. M. (1993).Proc. Natl. Acad. Sci. USA 90, 8653–8657.
Gill, I., and Vulfson, E. N. (1993).J. Am. Chem. Soc. 115, 3348–3349.
Gupta, M. N. (1992).Eur. J. Biochem. 203, 25–32.
Khan, S. A., Halling, P. J., Bosley, J. A., Clark, A. H., Peilow, A. D., Pelan, E. G., and Rowlands, D. W. (1992).Enzyme. Microb. Technol. 14, 96–100.
Khmelnitsky, Y. L., Levashov, A. V., Klyachko, N. L., and Martinek, K. (1988).Enzyme Microb. Technol. 10, 709–724.
Kise, H. (1990).Bioorg. Chem. 18, 107–115.
Kise, H., and Tomiuchi, Y. (1991).Biotech. Lett. 13, 317–322.
Kitaguchi, H., and Klibanov, A. M. (1989).J. Am. Chem. Soc. 111, 9272–9273.
Klibanov, A. M. (1986).Chemtech 16, 354–359.
Klibanov, A. M. (1989).Trends Biochem. Soc. 14, 141–144.
Klibanov, A. M. (1990).Acc. Chem. Res. 23, 114–120.
Kuhl, P., Halling, P. J., and Jakubke, H. D. (1990).Tetrahedron Lett. 31, 5213–5216.
Laane, C. (1987).Biocatalysis 1, 17–22.
Margolin, A. L., Tai, D. F., and Klibanov, A. M. (1987).J. Am. Chem. Soc. 109, 7885–7887.
Matsushima, A., Okada, M., and Inada, Y. (1994).FEBS Lett. 178, 275–277.
McPhalen, C. A., and James, M. N. G. (1988).Biochemistry 27, 6582–6598.
Merutka, G., Shalongo, W., and Stellwagen, E. (1991).Biochemistry 30, 4245–4248.
Nagashima, T., Watanabe, A., and Kise, H. (1992).Enzyme Microb. Technol. 14, 842–847.
Philipp, M., and Bender, M. L. (1983).Mol Cell. Biochem. 51, 5–32.
Phillips, R. S., Matthews, M. S., Olson, E., and Tersch, R. L. V. (1990).Enzyme Microb. Technol. 12, 731–735.
Reslow, M., Adlercreutz, P., and Mattiasson, B. (1988).Eur. J. Biochem. 172, 573–578.
Ricca, J. M., and Crout, D. H. G. (1989).J. Chem. Soc. Chem. Commun. 1989, 2126–2127.
Riva, S., Chopineau, J., Kieboom, A. P. G., and Klibanov, A. M. (1988).J. Am Chem. Soc. 110, 584–589.
Sakurai, T., Margolin, A. L., Russell, A. J., and Klibanov, A. M. (1988).J. Am. Chem. Soc. 110, 7236–7237.
Schellenberger, V., and Jakubke, H. D. (1991).Angew. Chem. Int. Ed. Engl. 30, 1437–1449.
Schultz, G. E., and Schirmer, R. H. (1979). InPrinciples of Protein Structure, Springer, New York.
Schulze, B., and Klibanov, A. M. (1991).Biotech. Bioeng. 38, 1001–1006.
Stahl, M., Mansson, M. O., and Mosbach, K. (1990).Biotech. Lett. 12, 161–166.
Waks, M. (1986).Proteins Struct. Funct. Genet,1, 4–15.
Wells, J. A., and Estell, D. A. (1988).Trends Biochem. Sci. 13, 291–294.
West, J. B., and Wong, C. H. (1986).J. Chem. Soc. Chem. Commun. 1986, 417–418.
Wong, C. H., Chen, S. T., Hennen, W. J., Bibbs, J. A., Wang, Y. F., Liu, J. L. C., Pantoliano, M. W., Whitlow, M., and Bryan, P. N. (1990).J. Am. Chem. Soc. 112, 945–953.
Wong, C. H., and Wang, K. T. (1991).Experientia 47, 1123–1129.
Zaks, A., and Klibanov, A. M. (1988).J. Biol. Chem. 263, 3194–3201.
Zhong, Z., Liu, J. J. C., Dinterman, L. M., Finkelman, M. A. J., Mueller, T. W., Rollence, M. L., Whitlow, M., and Wong, C. H. (1991).J. Am. Chem. Soc. 113, 683–684.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, ST., Chen, SY., Tu, CC. et al. Physicochemical properties of alkaline serine proteases in alcohol. J Protein Chem 14, 205–215 (1995). https://doi.org/10.1007/BF01886761
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01886761