Abstract
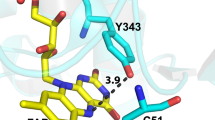
Phosphorescence and optically detected magnetic resonance (ODMR) measurements have been carried out on the tryptophan (Trp) residues ofEscherichia coli Trp repressor protein (W Rep) and its two single Trp-containing mutants, W19F and W99F. The enhanced resolution afforded by the W19F and W99F mutants allowed us to characterize the triplet state of boundl-Trp corepressor using phosphorescence wavelengt-selected ORMR spectroscopy. We find that at 77 K the 0,0 band peak wavelength ofl-Trp is shifted from 405.5 nm in the aqueous solvent to ca. 410 nm when bound to the corepressor binding site. This red shift of the phosphorescence along with a corresponding increase in the zero-field splittingE value and narrowing of the ODMR linewidth characterize a binding site that is less polar, as well as more polarizable and homogeneous, than the aqueous solvent. This conclusion is in agreement with the X-ray crystallographic structure of the holorepressor protein that places the indole chromophore of the bound corepressor in a cleft in which it is sandwiched by the side chains of arginines 54 and 84.
Similar content being viewed by others
References
J. K. Rose, C. L. Squires, C. Yanofsky, H.-L. Yang, and G. Zubay (1973)Nature New Biol. 245, 133–137.
R. P. Gunsalus and C. yanofsky (1980)Proc. Natl. Acad. Sci. USA 77, 7117–7121.
G. Zurawski, R. P. Gunsalus, K. D. Brown, and C. Yanofsky (1981)J. Mol. Biol. 145, 47–73.
R. L. Kelly and C. Yanofsky (1982)Proc. Natl. Acad. Sci. USA 79, 3120–3124.
A. Joachimiak, R. L. Kelly, R. P. Gunsalus, C. Yanofsky, and P. B. Sigler (1983)Proc. Natl. Acad. Sci. USA 80, 668–672.
A. N. Lane (1985)Eur. J. Biochem. 157, 405–413.
D. N. Arvidson, C. Bruce, and R. P. Gunsalus (1986)J. Biol. Chem. 261, 238–243.
R. Q. Marmorstein, A. Joachimiak, M. Sprinzl, and P. B. Sigler (1987)J. Biol. Chem. 262, 4922–4927.
I. P. Crawford and G. V. Stauffer (1980)Annu. Rev. Biochem. 49, 163–195.
T. Platt (1980) in J. H. Miller and W. S. Reznikoff (Eds.),The Operon, Ed 2, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp. 263–302.
R. L. Sommerville (1983) in K. M. Hermann and R. L. Sommerville (Eds.),Amino Acid Biosynthesis and Regulation, Addison-Wesley, Reading, MA, pp. 351–378.
R. W. Shevitz, Z. Otwinowski, A. Joachimiak, C. L. Lawson, and P. B. Sigler (1985)Nature 317, 782–786.
R.-G. Zhang, A. Joachimiak, C. L. Lawson, R.W. Shevitz, Z. Otwinowski, and P. B. Sigler (1987)Nature 327, 591–597.
Z. Otwinowski, R. W. Shevitz, R.-G. Zhang, C. L. Lawson, A. Joachimiak, R. Q. Marmorstein, B.F. Luisi, and P. B. Sigler (1988)Nature 335, 321–329.
C. L. Lawson, R.-G. Zhang, R. W. Shevitz, Z. Otwinowski, A. Joachimiak, and P. B. Sigler (1988)Proteins 3, 18–21.
R. Q. Marmorstein and P. B. Sigler (1989)J. Biol. Chem. 264, 9146–9154.
S. Arnott and D. W. L. Hukins (1972)Biochem. Biophys. Res. Commun. 47, 1504–1509.
M. R. Eftink, G. D. Ramsay, L. E. Burns, A. H. Maki, C. J. Mann, C. J. Matthews, and C. A. Ghiron (1993)Biochemistry 32, 9189–9198.
A. N. Lane and O. Jardetzky (1985)Eur. J. Biochem. 152, 411–418.
R. M. Purkey and W. C. Galley (1970)Biochemistry 9, 3569–3575.
J. M. Davis and A. H. Maki (1984)Biochemistry 23, 6249–6256.
J. G. Weers and A. H. Maki (1986)Biochemistry 25, 2897–2904.
S. Ghosh, L.-H. Zang, and A. H. Maki (1988)J. Chem. Phys. 88, 2769–2775.
J.-U. von Schütz, J. Zuclich, and A. H. Maki (1974)J. Am. Chem. Soc. 96, 714–718.
S.-Y. Mao and A. H. Maki (1987)Biochemistry 26, 3576–3582.
M. I. Khamis, J. R. Casas-Finet, A. H. Maki, J. B. Murphy, and J. W. Chase (1987)J. Biol. Chem. 262, 10938–10945.
S. Ghosh, L.-H. Zang, and A. H. Maki (1988)Biochemistry 27, 7816–7820.
A. H. Maki (1984) in L. J. Berliner and J. Reuben (Eds.),Biological Magnetic Resonance, Vol. 6, Plenum, New York, pp. 470–557.
A. J. Hoff (1989) in A. J. Hoff (Ed.),Advanced EPR with Applications in Biology and Biochemistry, Elsevier, Amsterdam, pp. 633–684.
D. W. Marquardt (1963)J. Soc. Indust. Appl. Math. 11, 431–441.
S. Ghosh, M. Petrin, and A. H. Maki (1986)Biophys. J. 49, 753–760.
H. C. Brenner and V. Kolubayev (1988)J. Lumin. 39, 251–257.
A. H. Maki, P. Svejda, and J. R. Huber (1978)Chem. Phys. 32, 369–380.
M. V. Hershberger, A. H. Maki, and W. C. Galley (1980)Biochemistry 19, 2204–2209.
W. C. Galley and R. M. Purkey (1970)Proc. Natl. Acad. Sci. 67, 1116–1121.
K. Itoh and T. Azumi (1973)Chem. Phys. 22, 395–399.
K. Itoh and T. Azumi (1975)J. Chem. Phys. 62, 3431–3438.
A. H. Maki and T. Co (1976)Biochemistry 15, 1229–1235.
C. L. Lawson and P. S. Sigler (1988)Nature 333, 869–871.
C. A. Royer, J. A. Gardner, J. M. Beechem, J.-C. Brochon, and K. S. Matthews (1990)Biophys. J. 58, 363–378.
J. van Egmond, B. E. Kohler, and I. Y. Chan (1975)Chem. Phys. Lett. 34, 423–426.
G. Gradl, J. Friedrich, and B. E. Kohler (1986)J. Chem. Phys. 84, 2079–2083.
J. Zuclich (1970)J. Chem. Phys. 52, 3586–3591.
J. Zuclich, D. Schweitzer, and A. H. Maki (1973)Photochem. Photobiol. 18, 161–168.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Burns, L.E., Maki, A.H. Study ofl-tryptophan corepressor binding to mutatedE. coli tryptophan repressor proteins by optically detected triplet-state magnetic resonance. J Fluoresc 4, 217–226 (1994). https://doi.org/10.1007/BF01878454
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01878454