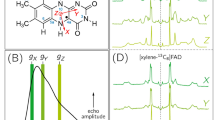
Abstract
EPR spectroscopy is an important spectroscopic method for identification and characterization of radical species involved in many biological reactions. The tyrosyl radical is one of the most studied amino acid radical intermediates in biology. Often in conjunction with histidine residues, it is involved in many fundamental biological electron and proton transfer processes, such as in the water oxidation in photosystem II. As biological processes are typically extremely complicated and hard to control, molecular bio-mimetic model complexes are often used to clarify the mechanisms of the biological reactions. Here, we present theoretical calculations to investigate the sensitivity of magnetic resonance parameters to proton-coupled electron transfer events, as well as conformational substates of the molecular constructs which mimic the tyrosine–histidine (Tyr–His) pairs found in a large variety of proteins. Upon oxidation of the phenol, the Tyr analog, these complexes can perform not only one-electron one-proton transfer (EPT), but also one-electron two-proton transfers (E2PT). It is shown that in aprotic environment the gX-components of the electronic g-tensor are extremely sensitive to the first proton transfer from the phenoxyl oxygen to the imidazole nitrogen (EPT product), leading to a significant increase of the gX-value of up to 0.003, but are not sensitive to the second proton transfer (E2PT). In the latter case, the change of the gX-value is much smaller (ca. 0.0001), which is too small to be distinguished even by high-frequency EPR. The 14N hyperfine values are also too similar to allow differentiation between the different protonation states in EPT and E2PT. The magnetic resonance parameters were also calculated as a function of the rotation angles around single bonds. It was demonstrated that rotation of the phenoxyl group results in large positive changes (> 0.001) in the gX-values. Analysis of the data reveals that the main source of these changes is related to the strength of the H-bond between phenoxyl oxygen and the proton(s) on N1 and N2 positions of the imidazole.





Similar content being viewed by others
References
K.-P. Dinse, G. Jeschke, Methods in Physical Chemistry (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2012), pp. 159–189
D. Goldfarb, S. Stoll (eds.), EPR Spectroscopy: Fundamentals and Methods (Wiley, New York, 2018)
S.K. Misra (ed.), Multifrequency Electron Paramagnetic Resonance: Theory and Applications (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2011)
K. Möbius, A. Savitsky, High-Field EPR Spectroscopy on Proteins and Their Model Systems: Characterization of Transient Paramagnetic States (The Royal Society of Chemistry, Cambridge, 2009)
A. Lund, M. Shiotani, S. Shimada, Principles and Applications of ESR Spectroscopy (Springer, Dordrecht, 2011)
A. Schweiger, G. Jeschke, Principles of Pulse Electron Paramagnetic Resonance (Oxford University Press, New York, 2001)
J.A. Weil, J.R. Bolton, J.A. Weil, J.R. Bolton, J.E. Wertz, Electron Paramagnetic Resonance: Elementary Theory and Practical Applications (Wiley, New York, 2007)
M. Drescher, G. Jeschke (eds.), EPR Spectroscopy: Applications in Chemistry and Biology (Springer, Berlin, 2012)
G.R. Eaton, S.S. Eaton, K.M. Salikhov (eds.), Foundations of Modern EPR (World Scientific Publishing, Singapore, 1998)
J. Niklas, O.G. Poluektov, Adv. Energy Mater. 7, 1602226 (2017)
K. Möbius, W. Lubitz, N. Cox, A. Savitsky, Magnetochemistry 4, 85 (2018)
F. Gerson, W. Huber, Electron Spin Resonance Spectroscopy of Organic Radicals (Wiley-VCH, Weinheim, 2003)
H. Kurreck, B. Kirste, W. Lubitz, Angew. Chem. Int. Edit. 23, 173–194 (1984)
H. Kurreck, B. Kirste, W. Lubitz, Electron Nuclear Double Resonance Spectroscopy of Radicals in Solution—Applications to Organic and Biological Chemistry (VCH Publishers Inc., Deerfield Beach, 1988)
K. Möbius, W. Fröhling, F. Lendzian, W. Lubitz, M. Plato, C.J. Winscom, J. Phys. Chem. 86, 4491–4507 (1982)
G. Jeschke, ChemPhysChem 3, 927–932 (2002)
C. Gemperle, A. Schweiger, Chem. Rev. 91, 1481–1505 (1991)
A. Savitsky, K. Möbius, Photosynth. Res. 102, 311–333 (2009)
O. Grinberg, L.J. Berliner (eds.), Very High Frequency (VHF) ESR/EPR, vol. 22 (Springer, New York, 2004)
L. Kulik, W. Lubitz, Photosynth. Res. 102, 391–401 (2009)
Y.S. Lebedev, Appl. Magn. Reson. 7, 339–362 (1994)
O.Y. Grinberg, A.A. Dubinskii, in Very High Frequency (VHF) ESR/EPR, vol. 22, ed. by O. Grinberg, L.J. Berliner (Springer, New York, 2004), pp. 1–18
A.A. Galkin, O.Y. Grinberg, A.A. Dubinskii, N.N. Kabdin, V.N. Krymov, V.I. Kurochkin, Y.S. Lebedev, L.F. Oranskii, V.F. Shuvalov, Instrum. Exp. Tech. 20, 1229–1229 (1977)
A.Y. Bresgunov, A.A. Dubinskii, V.N. Krimov, Y.G. Petrov, O.G. Poluektov, Y.S. Lebedev, Appl. Magn. Reson. 2, 715–728 (1991)
O.Y. Grinberg, A.A. Dubinskii, Y.S. Lebedev, Usp. Khim. 52, 1490–1513 (1983)
C. Rudowicz, S.K. Misra, Appl. Spectrosc. Rev. 36, 11–63 (2001)
F. Neese, Multifrequency Electron Paramagnetic Resonance (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2011), pp. 295–326
M. Kaupp, M. Bühl, V.G. Malkin (eds.), Calculation of NMR and EPR Parameters: Theory and Applications (Wiley-VCH, Weinheim, 2004)
F. Neese, in High Resolution EPR: Applications to Metalloenzymes and Metals in Medicine, ed. by L. Berliner, G. Hanson (Springer New York, New York, 2009), pp. 175–229
F. Neese, Coord. Chem. Rev. 253, 526–563 (2009)
M.L. Munzarova, P. Kubacek, M. Kaupp, J. Am. Chem. Soc. 122, 11900–11913 (2000)
M. Munzarova, M. Kaupp, J. Phys. Chem. A 103, 9966–9983 (1999)
M. Retegan, N. Cox, W. Lubitz, F. Neese, D.A. Pantazis, Phys. Chem. Chem. Phys. 16, 11901–11910 (2014)
G.T. Babcock, M. Espe, C. Hoganson, N. LydakisSimantiris, J. McCracken, W.J. Shi, S. Styring, C. Tommos, K. Warncke, Acta Chem. Scand. 51, 533–540 (1997)
D.L. Jenson, B.A. Barry, J. Am. Chem. Soc. 131, 10567–10573 (2009)
B.A. Barry, Biochim. Biophys. Acta Bioenerg. 1847, 46–54 (2015)
S. Un, P. Dorlet, A.W. Rutherford, Appl. Magn. Reson. 21, 341–361 (2001)
P. Faller, C. Goussias, A.W. Rutherford, S. Un, Proc. Natl. Acad. Sci. USA 100, 8732–8735 (2003)
R.E. Blankenship, Molecular Mechanisms of Photosynthesis (Blackwell Science Limited, Oxford, 2002)
T.J. Wydrzynski, K. Satoh (eds.), Photosystem II—The Light-Driven Water: Plastoquinone Oxidoreductase, vol. 22 (Springer, Dordrecht, 2005)
Y. Umena, K. Kawakami, J.R. Shen, N. Kamiya, Nature 473, 55–60 (2011)
P. Faller, R.J. Debus, K. Brettel, M. Sugiura, A.W. Rutherford, A. Boussac, Proc. Natl. Acad. Sci. USA 98, 14368–14373 (2001)
R. Chatterjee, C.S. Coates, S. Milikisiyants, C.I. Lee, A. Wagner, O.G. Poluektov, K.V. Lakshmi, Biochemistry 52, 4781–4790 (2013)
S.J. Mora, E. Odella, G.F. Moore, D. Gust, T.A. Moore, A.L. Moore, Acc. Chem. Res. 51, 445–453 (2018)
J.D. Megiatto, D.D. Mendez-Hernandez, M.E. Tejeda-Ferrari, A.L. Teillout, M.J. Llansola-Portoles, G. Kodis, O.G. Poluektov, T. Rajh, V. Mujica, T.L. Groy, D. Gust, T.A. Moore, A.L. Moore, Nat. Chem. 6, 423–428 (2014)
D.A. Svistunenko, Biochim. Biophys. Acta Bioenerg. 1707, 127–155 (2005)
A. Migliore, N.F. Polizzi, M.J. Therien, D.N. Beratan, Chem. Rev. 114, 3381–3465 (2014)
J. Stubbe, W.A. van der Donk, Chem. Rev. 98, 705–762 (1998)
L. Hammarström, S. Styring, Energy Environ. Sci. 4, 2379–2388 (2011)
H.B. Gray, J.R. Winkler, Acc. Chem. Res. 51, 1850–1857 (2018)
S.Y. Reece, D.G. Nocera, Annu. Rev. Biochem. 78, 673–699 (2009)
E. Odella, S.J. Mora, B.L. Wadsworth, J.J. Goings, M.A. Gervaldo, L.E. Sereno, T.L. Groy, D. Gust, T.A. Moore, G.F. Moore, S. Hammes-Schiffer, A.L. Moore, Chem. Sci. 11, 3820–3828 (2020)
E. Odella, B.L. Wadsworth, S.J. Mora, J.J. Goings, M.T. Huynh, D. Gust, T.A. Moore, G.F. Moore, S. Hammes-Schiffer, A.L. Moore, J. Am. Chem. Soc. 141, 14057–14061 (2019)
E. Odella, S.J. Mora, B.L. Wadsworth, M.T. Huynh, J.J. Goings, P.A. Liddell, T.L. Groy, M. Gervaldo, L.E. Sereno, D. Gust, T.A. Moore, G.F. Moore, S. Hammes-Schiffer, A.L. Moore, J. Am. Chem. Soc. 140, 15450–15460 (2018)
M.T. Huynh, S.J. Mora, M. Villalba, M.E. Tejeda-Ferrari, P.A. Liddell, B.R. Cherry, A.L. Teillout, C.W. Machan, C.P. Kubiak, D. Gust, T.A. Moore, S. Hammes-Schiffer, A.L. Moore, ACS Cent. Sci. 3, 372–380 (2017)
P.J. Stephens, F.J. Devlin, C.F. Chabalowski, M.J. Frisch, J. Phys. Chem. 98, 11623–11627 (1994)
A.D. Becke, J. Chem. Phys. 98, 5648–5652 (1993)
J. Baker, T. Janowski, K. Wolinski, P. Pulay, Wiley Interdiscip. Rev. Comput. Mol. Sci. 2, 63–72 (2012)
S.G. Balasubramani, G.P. Chen, S. Coriani, M. Diedenhofen, M.S. Frank, Y.J. Franzke, F. Furche, R. Grotjahn, M.E. Harding, C. Hattig, A. Hellweg, B. Helmich-Paris, C. Holzer, U. Huniar, M. Kaupp, A.M. Khah, S.K. Khani, T. Müller, F. Mack, B.D. Nguyen, S.M. Parker, E. Perlt, D. Rappoport, K. Reiter, S. Roy, M. Rückert, G. Schmitz, M. Sierka, E. Tapavicza, D.P. Tew, C. Wüllen, V.K. Voora, F. Weigend, A. Wodyński, J.M. Yu, J. Chem. Phys. 152, 36 (2020)
F. Weigend, R. Ahlrichs, Phys. Chem. Chem. Phys. 7, 3297–3305 (2005)
F. Furche, R. Ahlrichs, C. Hattig, W. Klopper, M. Sierka, F. Weigend, Wiley Interdiscip. Rev. Comput. Mol. Sci. 4, 91–100 (2014)
S. Grimme, S. Ehrlich, L. Goerigk, J. Comput. Chem. 32, 1456–1465 (2011)
S. Grimme, J. Antony, S. Ehrlich, H. Krieg, J. Chem. Phys. 132, 154104 (2010)
F. Neese, Wiley Interdiscip. Rev. Comput. Mol. Sci. 8, 6 (2018)
F. Neese, Wiley Interdiscip. Rev. Comput. Mol. Sci. 2, 73–78 (2012)
V. Barone, M. Cossi, J. Phys. Chem. A 102, 1995–2001 (1998)
M. Steinmetz, A. Hansen, S. Ehrlich, T. Risthaus, S. Grimme, in Density Functionals: Thermochemistry, ed. by E.R. Johnson (Springer International Publishing, Cham, 2015), pp. 1–23
D. Svistunenko, M. Adelusi, M. Dawson, P. Robinson, C. Bernini, A. Sinicropi, R. Basosi, Stud. Univ. Babes Bolyai Chem. 56, 135–146 (2011)
D.A. Svistunenko, C.E. Cooper, Biophys. J. 87, 582–595 (2004)
S. Sinnecker, E. Reijerse, F. Neese, W. Lubitz, J. Am. Chem. Soc. 126, 3280–3290 (2004)
G.J. Gerfen, B.F. Bellew, S. Un, J.M. Bollinger, J. Stubbe, R.G. Griffin, D.J. Singel, J. Am. Chem. Soc. 115, 6420–6421 (1993)
J.R. Asher, M. Kaupp, Theor. Chem. Acc. 119, 477–487 (2008)
J.R. Asher, N.L. Doltsinis, M. Kaupp, Magn. Reson. Chem. 43, S237–S247 (2005)
J.R. Asher, N.L. Doltsinis, M. Kaupp, J. Am. Chem. Soc. 126, 9854–9861 (2004)
S. Un, M. Atta, M. Fontecave, A.W. Rutherford, J. Am. Chem. Soc. 117, 10713–10719 (1995)
L. Benisvy, R. Bittl, E. Bothe, C.D. Garner, J. McMaster, S. Ross, C. Teutloff, F. Neese, Angew. Chem. Int. Edit. 44, 5314–5317 (2005)
T.I. Smirnova, A.I. Smirnov, S.V. Paschenko, O.G. Poluektov, J. Am. Chem. Soc. 129, 3476–3477 (2007)
T.I. Smirnova, T.G. Chadwick, M.A. Voinov, O. Poluektov, J. van Tol, A. Ozarowski, G. Schaaf, M.M. Ryan, V.A. Bankaitis, Biophys. J. 92, 3686–3695 (2007)
K. Möbius, A. Savitsky, C. Wegener, M. Plato, M. Fuchs, A. Schnegg, A.A. Dubinskii, Y.A. Grishin, I.A. Grigor'ev, M. Kuhn, D. Duché, H. Zimmermann, H.J. Steinhoff, Magn. Reson. Chem. 43, S4–S19 (2005)
M. Plato, H.J. Steinhoff, C. Wegener, J.T. Torring, A. Savitsky, K. Möbius, Mol. Phys. 100, 3711–3721 (2002)
S. Sinnecker, A. Rajendran, A. Klamt, M. Diedenhofen, F. Neese, J. Phys. Chem. A 110, 2235–2245 (2006)
M. Witwicki, J. Jezierska, A. Ozarowski, Chem. Phys. Lett. 473, 160–166 (2009)
Acknowledgements
This study is based upon work supported by U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences, under contract number DE-AC02-06CH11357 at Argonne National Laboratory (JN and OGP) and DE-FG02-03ER15393 (ALM and TAM). The work was supported by the Illinois Space Grant Consortium and National Institutes of Health (SC3 GM122614) (HO and KLM). We gratefully acknowledge the computing resources provided on Blues and Bebop, high-performance computing clusters operated by the Laboratory Computing Resource Center at Argonne National Laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mardis, K.L., Niklas, J., Omodayo, H. et al. One Electron Multiple Proton Transfer in Model Organic Donor–Acceptor Systems: Implications for High-Frequency EPR. Appl Magn Reson 51, 977–991 (2020). https://doi.org/10.1007/s00723-020-01252-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-020-01252-8