Summary
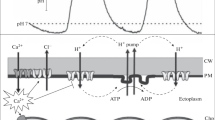
We previously introduced a flash spectrophotometric method to analyze proton conduction by CF0 in vesicles derived from thylakoid membranes (H. Lill, S. Engelbrecht, G. Schönknecht & W. Junge, 1986,Eur. J. Biochem. 160:627–634). The unit conductance of CF0, as revealed by this technique, was orders of magnitude higher than that theoretically expected for a hydrogen-bonded chain. We scrutinized the validity of this method. Small vesicles were derived from thylakoids by EDTA treatment. The intrinsic electric generators in the membrane were stimulated by short flashes of light and the relaxation of the voltage via ionic channels was measured through electrochromic absorption changes of intrinsic pigments. The voltage decay was stimulated by a statistical model. As the vesicle-size distribution had only a minor influence, the simulation required only two fit parameters, the first proportional to the unit conductance of an active channelG, and the second denoting the average number of active channels per vesiclen. This technique was applied to CF0, the proton channel of the chloroplast ATP synthase, and to gramicidin, serving as a standard. For both channels we found the above two fit parameters physically meaningful. They could be independently varied in predictable wasy, i.e.n by addition of known inhibitors of F0-type proton channels andG via the temperature. for gramicidin, the unit conductance (2.7 pS) was within the range described in the literature. This established the competence of this method for studies on the mechanism of proton conduction by CF0, whose conductance so far has not been accessible to other, more conventional approaches. The time-averaged unit conductance of CF0 was about 1 pS, equivalent to the turnover of 6×105 H+/(CF0·sec) at 100 mV driving force.
Similar content being viewed by others
References
Apell, H.J., Bamberg, E., Läuger, P. 1979. Effects of surface charge on the conductance of the gramicidin channel.Biochim. Biophys. Acta 552:369–378
Apell, H.J., Läuger, P. 1986. Quantitative analysis of pumpmediated fluxes in reconstituted lipid vesicles.Biochim. Biophys. Acta 861:302–310
Bamberg, E., Läuger, P. 1974. Temperature dependent properties of gramicidin A channels.Biochim. Biophys. Acta 367:127–133
Brünger, A., Schulten, Z., Schulten, K. 1983. A network thermodynamic investigation of stationary and non-stationary proton transport through proteins.Z. Phys. Chem. 136:1–63
Caceci, M.S., Cacheris, W.P. 1984. Fitting curves to data. The simplex algorithm is the answer.Byte Mag. 9:340–362
Cain, K., Partis, M.D., & Griffiths, D.E. 1977. Dibutylchloromethyltin chloride, a covalent inhibitor of the adenosine triphosphate synthase complex.Biochem. J. 166:593–602
Cain, B.D., Simoni, R.D. 1986. Impaired proton conductivity resulting from mutations in thea subunit of F1F0ATPase inEscherichia coli.J. Biol. Chem. 261:10043–10050
Cox, G.B., Fimmel, A.L., Gibson, F., Hatch, L. 1986. The mechanism of ATP synthase: A reassessment of the functions of theb anda subunits.Biochim. Biophys. Acta 849:62–69
Davenport, J.W., McCarty, R.E. 1981. Quantitative aspects of adenosine triphosphate-driven proton translocation in spinach chloroplast thylakoids.J. Biol. Chem. 256:8947–8954
Finkelstein, A., Andersen, O.S. 1981. The gramicidin A channel: A review of its permeability characteristics with special reference to the single-file aspect of transport.J. Membrane Biol. 59:155–171
Förster V., Hong, Y.Q., Junge, W. 1981. Electron transfer and proton pumping under excitation of dark-adapted chloroplasts with flashes of light.Biochim. Biophys. Acta 638:141–152
Friedl, P., Schairer, H.U. 1981. The isolated F0 ofEscherichia coli ATP-synthase is reconstitutively active in H+-conduction and ATP-dependent energy-transduction.FEBS Lett. 128:261–264
Gould, J.M. 1976. Inhibition by triphenyltin chloride of a tightlybound membrane component involved in photophosphorylation.Eur. J. Biochem. 62:567–575
Hille, B. 1984. Ionic Channels of Excitable Membranes. Sinauer Associated, Inc., Sunderland, Mass.
Hladky, S.B., Haydon, D.A. 1973. Membrane conductance and surface potential.Biochim. Biophys. Acta 318:464–468
Hong, Y.Q., Junge, W. 1983. Localized or delocalized protons in photophosphorylation? On the accessibility of the thylakoid lumen for ions and buffers.Biochem. Biophys. Acta 722:197–208
Hoppe, J., Sebald, W. 1984. The proton conducting F0-part of bacterial ATP synthases.Biochem. Biophys. Acta 768:1–27
Itoh, S. 1979. Surface potential and reaction of the membrane-bound electron transfer components. II. Integrity of the chloroplast membrane and reaction of P-700.Biochim. Biophys. Acta 548:596–607
Jackson, J.B., Crofts, A.R. 1969. High energy state in chromatophores ofRhodopseudomonas spheroides.FEBS Lett. 4:185–189
Junesch, U., Gräber, P. 1985. The rate of ATP synthesis as a function of ΔpH in normal and dithiotheritol-modified chloroplasts.Biochim. Biophys. Acta 809:429–434
Junge, W. 1976. Flash kinetics spectrophotometry in the study of plant pigments.In: Chemistry and Biochemistry of Plant Pigments. pp. 233–333. T.W. Goodwin, editor. Vol. II, Academic, New York
Junge, W. 1982. Electrogenic reactions and proton pumping in green plant photosynthesis.Curr. Top. Membr. Transp. 16:431–465
Junge, W., Jackson, B. 1982. The development of electrochemical potential gradients across photosynthetic membranes.In: Photosynthesis: Energy Conversion by Plants and Bacteria. pp. 589–646. Govindjee, editor. Vol. II, Academic, New York
Junge, W., Rumberg, B., Schröder, H. 1970. The necessity of an electric potential difference and its use for photophosphorylation in short flash groups.Eur. J. Biochem. 14:575–581
Junge, W., Witt, H.T. 1968. On the ion transport system of photosynthesis. Investigations on a molecular level.Z. Naturforsch. 38:244–254
Kumamoto, C.A., Simoni, R.D. 1986. Genetic evidence for interaction between thea andb subunits of the F0 portion of theEscherichia coli proton translocating ATPase.J. Biol. Chem. 261:10037–10042
Lill, H., Engelbrecht, S., Schönknecht, G., Junge, W. 1986. The proton channel, CF0, of thylakoid membranes. Only a low proportion of CF1-lacking CF0 is active with a high unit conductance (169 fS).Eur. J. Biochem. 160:627–634
McLaughlin, S. 1977. Electrostatic potentials at membrane-solution interfaces.Curr. Top. Membr. Transp. 9:71–144
Mitchell, P. 1961. Coupling of phosphorylation of electron and hydrogen transfer by a chemiosmotic type of mechanism.Nature (London) 191:144–148
Mitchell, P. 1977. A commentary on alternative hypotheses of protonic coupling in the membrane systems catalysing oxidative and photosynthetic phosphorylation.FEBS Lett. 78:1–20
Mitchell, P. 1985. Molecular mechanics of protonmotive F0F1ATPases Rolling well and turnstile hypothesis.FEBS Lett. 182:1–7
Nagle, J.F., Tristram-Nagle, S. 1983. Hydrogen bonded chain mechanisms for proton conduction and proton pumping.J. Membrane Biol..74:1–14
Negrin, R.S., Foster, D.L., Fillingame, R.H. 1980. Energy-transducing H+-ATPase ofEscherichia coli. Reconstitution of proton translocating activity of the intrinsic membrane sector.J. Biol. Chem. 255:5643–5648
Neher, E., Sakmann, B. 1976. Single-channel currents recorded from membrane of denervated frog muscle fibers.Nature (London) 260:779–802
Polle, A., Junge, W. 1986. The slow rise of the flash-induced alkalization by photosystem II of the suspending medium is reversibly related to thylakoid stacking.Biochim. Biophys. Acta 848:257–264
Rumberg, B., Muhle, H. 1976. Investigation of the kinetics of proton translocation across the thylakoid membrane.Bioelectrochem. Bioenerget. 3:393–403
Schmid, R., Junge, W. 1975. Current-voltage studies on the thylakoid membrane in the presence of ionophores.Biochim. Biophys. Acta 394:76–92
Schneider, E., Altendorf, K. 1982. ATP synthetase (F1F0) ofEscherichia coli K-12. High-yield preparation of functional F0 by hydrophobic affinity chromatography.Eur. J. Biochem. 126:149–153
Schönknecht, G., Junge, W., Lill, H., Engelbrecht, S. 1986. Complete tracking of proton flow in thylakoids—The unit conductance of CF0 is greater than 10 fS.FEBS Lett. 203:289–294
Sigrist-Nelson, K., Sigrist, H., Azzi, A. 1978. Characterization of the dicyclohexylcarbodiimide binding protein isolated from chloroplast membranes.Eur. J. Biochem. 92:9–14
Sone, N., Hamamoto, T., Kagawa, Y. 1981. pH dependence of H+ conduction through the membrane moiety of the H+-ATPase (F0·F1) and effects of tyrosyl residue modification.J. Biol. Chem. 256:2873–2877
Szabo, G., Eisenman, G., McLaughlin, S.G.A., Krasne, S. 1972. Ionic probes of membrane structure.Ann. N. Y. Acad. Sci. 195:273–290
Thomas, J.B., Minnaert, K., Elbers, P.D. 1956. Chlorophyll concentration in plastids of different groups of plants.Acta Bot. Neerl. 5:314–321
Urry, D.W., Goodall, M.C., Glickson, J., Mayers, D.F. 1971. The gramicidin A transmembrane channel: Characterics of head-to-head dimerized π(L,D) helices.Proc. Natl. Acad. Sci. USA 68:1907–1911
Vignais, P.V., Satre, M. 1984. Recent developments on structural and functional aspects of the F1 sector of H+-linked ATPases.Mol. Cell. Biochem. 60:33–70
Witt, H.T. 1975. Primary acts of energy conservation in the functional membrane of photosynthesis.In: Bioenergetics of Photosynthesis. Govindjee, editor. pp. 493–554. Academic, New York
Witt, H.T. 1979. Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. The central role of the electric field.Biochim. Biophys. Acta 505:355–427
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lill, H., Althoff, G. & Junge, W. Analysis of ionic channels by a flash spectrophotometric technique applicable to thylakoid membranes: CF0, the proton channel of the chloroplast ATP synthase, and, for comparison, gramicidin. J. Membrain Biol. 98, 69–78 (1987). https://doi.org/10.1007/BF01871046
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01871046