Summary
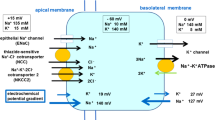
The effect of sulfhydryl reagents on the Na+ permeability mechanisms of toad urinary bladder vesicles was examined. The reagents 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB), iodosobenzoate, and ethylenimine were able to decrease amiloride-inhibited sodium uptake into vesicles when used at low concentrations. When used at higher concentrations these reagents were able to induce large increases in vesicle Na+ permeability that were not sensitive to amiloride. The reagentp-chloro-mercuribenzene sulfonate was able to induce such leaks even at low concentrations. The reagent N-ethylmaleimide was incapable of substantially affecting vesicle Na+ transport in any way. All of the effects observed could be reversed by removing the reagents from the solution surrounding the vesicles. Our results help explain the varied actions of sulfhydryl reagents on intact epithelial tissue.
Similar content being viewed by others
References
Benos, D.J., Mandel, L.J., Simon, S.A. 1980. Effects of chemical group specific reagents on sodium entry and the amiloride binding site in frog skin: Evidence for separate sites.J. Membrane Biol. 56:149–158
Brown, K.M., Dennis, J.E. 1972. Derivative-free analogues of the Levenberg-Marquardt and Gauss algorithms for nonlinear least squares approximation.Numer. Math. 18:289–297
Dick, H.J., Lindemann, B. 1975. Saturation of Na-current into frog skin epithelium abolished by PCMB.Pflueger's Arch. 355:R72
Ellman, G.L. 1959. Tissue sulfhydryl groups.Arch. Biochem. Biophys. 82:70–77
Ferreira, K.T.G. 1970. The effect of Cu2+ on isolated frog skin.Biochim. Biophys. Acta 203:555–567
Fleisher, L.N., Yorio, T., Bentley, P.J. 1975. Effect of cadmium on epithelial membranes.Toxicol. Appl. Pharmacol. 33:384–387
Frenkel, A., Ekblad, E.B.M., Edelman, I.S. 1975. Effects of sulfhydryl reagents on basal and vasopressin-stimulated Na+ transport in the toad bladder.In: Biomembranes. H. Eisenberg, E. Katchalski-Katzir, and L.A. Manson, editors. Vol. 7, pp. 61–80. Plenum, New York
Godin, D.V., Schrier, S.L. 1972. Modification of the erythrocyte membrane by sulfhydryl group reagents.J. Membrane Biol. 7:285–312
Harms, V., Fanestil, D.D. 1977. Functions of apical membrane of toad urinary bladder: Effects of membrane impermeant reagents.Am. J. Physiol. 233:F607-F614
Hillyard, S.D., Gonick, H.C. 1976. Effects of Cd++ on shortcircuit current across epithelial membranes. I. Interactions with Ca++ and vasopressin on frog skin.J. Membrane Biol. 26:109–119
Janatova, J., Fuller, J.K., Hunter, M.J. 1968. The heterogeneity of bovine albumin with respect to sulfhydryl and dimer content.J. Biol. Chem. 243(13):3612–3622
Knauf, P.A., Rothstein, A. 1971. Chemical modification of membranes: I. Effects of sulfhydryl and amino reactive reagents on anion and cation permeability of the human red blood cell.J. Gen. Physiol. 58:190–210
LaBelle, E.F., Valentine, M.E. 1980. Inhibition by amiloride of22Na+ transport into toad bladder microsomes.Biochim. Biophys. Acta 601:195–205
Li, J.H., Sousa, R.C. de 1977. Effects of Ag+ on frog skin: Interactions with oxytocin, amiloride and ouabain.Experientia 33(4):433–436
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. 1951. Protein measurement with the Folin phenol reagent.J. Biol. Chem. 193:265–275
Means, G., Feeney, R.E. 1971. Chemical Modification of Proteins. p. 156ff. Holden Day, San Franciso
Schaeffer, J.F., Preston, R.L., Curran, P.F. 1973. Inhibition of amino acid transport in rabbit intestine byp-chloromercuriphenyl sulfonic acid.J. Gen. Physiol. 62:131–146
Spooner, P.M., Edelman, I.S. 1976. Stimulation of Na+ transport across the toad urinary bladder byp-chloromercuribenzene sulfonate.Biochim. Biophys. Acta 455:272–276
Stymans, A., Van Driessche, W., Borghgraef, R. 1973. Multivalent cations and anionic substitution effects on frog skin.Arch. Int. Physiol. Biochim. 81:166–168
Sutherland, R.M., Rothstein, A., Weed, R.I. 1967. Erythrocyte membrane sulfhydryl groups and cation permeability.J. Cell. Physiol. 69:185–198
Will, P.C., Hopfer, U. 1979. Apparent inhibition of active nonelectrolyte transport by an increased sodium permeability of the plasma membrane.J. Biol. Chem. 254(10):3806–3811
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
LaBelle, E.F., Eaton, D.C. Sulfhydryl reagents affect Na+ uptake into toad bladder membrane vesicles. J. Membrain Biol. 71, 39–45 (1983). https://doi.org/10.1007/BF01870673
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01870673