Abstract
The rate of sodium current decay at −140 mV was studied as a function of the duration and amplitude of the activating voltage pulse. These sodium current decays or tails of current showed a biexponential decline in amplitude which depended upon the duration of the activating pulse. At 12°C, the two exponential components of the Na tail currents exhibited time constants of 72 and 534 μs. As the duration of an activating pulse was lengthened, the relative amplitude of the slow component of the decay increased compared to the fast component, without any changes in the fast and slow time constants. This slowing of the decay of current as a function of the duration of the activating pulse is found only in fibers with inactivation intact.
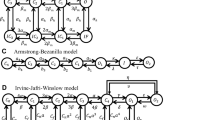
A number of Markov models were tested for their ability to predict the biexponential decays found in muscle fibers with inactivation intact and removed. A homogeneous population of channels having only a single open state fails to predict the behavior. A homogeneous population of channels having two open states predicts the behavior. The behavior can also be predicted by two different types of single open-state sodium channels, with one ensemble of channels carrying a minority of the current and exhibiting a much slower closing rate. If a homogeneous population of channels is present, the simulations show that the observed changes in decay rates are driven by inactivation.
Similar content being viewed by others
References
Aldrich, R. W., D. P. Corey, and C. F. Stevens. 1983. A reinterpretation of mammalian sodium channel gating based on single channel recording.Nature 306: 436–441.
Campbell, D. T. 1983. Sodium channel gating currents in frog skeletal muscle.J. Gen. Physiol. 82: 679–701.
Campbell, D. T. and R. Hahin. 1983. Altered sodium and gating currents in frog skeletal muscle caused by low external pH.J. Gen. Physiol. 84: 771–788.
Frankenhaeuser, B. and A. L. Hodgkin. 1957. The action of calcium on the electrical properties of squid giant axons.J. Physiol. (Lond.) 137: 218–244.
Gilly, W. F. and C. M. Armstrong. 1984. Threshold channels —A novel type of sodium channel in squid giant axon.Nature 309: 448–450.
Goldman, L. and R. Hahin. 1978. Initial conditions and the kinetics of the sodium conductance inMyxicola giant axons.J. Gen. Physiol. 72: 879–898.
Hahin, R. 1987. Removal of inactivation causes time-invariant Na current decays.Biophys. J. 51: 439a.
Hahin, R. 1988. Removal of inactivation causes time-invariant sodium current decay rates.J. Gen. Physiol. 92: 331–350.
Hahin, R. and D. T. Campbell. 1983. Simple shifts in the voltage dependence of sodium channel gating caused by divalent cations.J. Gen. Physiol. 82: 785–805.
Hille, B. and D. T. Campbell. 1976. An improved vaseline gap voltage clamp for sekeletal muscle fibers.J. Gen. Physiol. 67: 265–293.
Hodgkin, A. L. and A. F. Huxley. 1952. The components of membrane conductance in the giant axon ofLoligo.J. Physiol. (Lond.) 116: 473–496.
Horn, R. and C. A. Vandenberg. 1984. Statistical properties of single sodium channels.J. Gen. Physiol. 84: 505–534.
Mozhaeva, G. N., A. P. Naumov, and E. D. Nosyreva. 1980. Kinetics of sodium tail current during repolarization of axon membrane normally and in the presence of scorpion toxin.Neirofiziol. 12: 541–549.
Neher, E. and B. Sakmann. 1976. Single-channel currents recorded from membrane of denervated frog muscle fibres.Nature 260: 799–801.
Pappone, P. A. 1980. Voltage-clamp experiments in normal and denervated mammalian skeletal muscle fibres.J. Physiol. (Lond.) 306: 377–410.
Sevcik, C. 1976 Binding of tetrodotoxin to squid nerve fibers. Two kinds of receptors.J. Gen. Physiol. 68: 95–103.
Sigworth, F. J. 1980. Covariance of nonstationary sodium current fluctuations at the node of Ranvier.Biophys. J. 34: 111–133.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hahin, R. Two open states or two different Na channels in skeletal muscle fibers: Markov models of the decay of Na currents in frog skeletal muscle. J Biol Phys 16, 81–92 (1988). https://doi.org/10.1007/BF01867370
Issue Date:
DOI: https://doi.org/10.1007/BF01867370