Summary
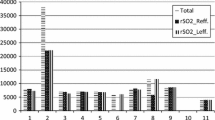
The present study compares the change of cerebral blood flow and HbO2 measured by near-infrared spectroscopy (NIRS) after administration of 1000 mg acetazolamide intravenously. CBF studies in 21 patients with ischaemic cerebrovascular disease were performed routinely with the133Xenon technique. Additionally the local HbO2 was recorded by NIRS. A rest study was followed by a second study after the administration of 1000 mg acetazolamide. In 18 patients we observed an increase of 30.8% of CBF and 4.7% of HbO2, 3 patients showed a decrease of CBF and 2 patients a simultaneous decrease of HbO2. We did not find a correlation between the absolute values of CBF and HbO2 at rest or after stimulation. However, a positive correlation (r=0.71, p < 0.05) between the change of CBF and HbO2 could be detected. Assuming a threshold value of normal CBF reactivity of 30% and 4% HbO2 reactivity we found for NIRS a sensitivity of 0.88 and a specificity of 0.75.
The results demonstrate that changes of CBF can be detected with NIRS and the algorithm of the used monitor is able to calculate the intracranial part of the signal. So, NIRS can be used as non-invasive screening method to test the cerebrovascular reserve capacity.
Similar content being viewed by others
References
Ausman JI, McCormick PW, Stewart M, Lewis G, Dujovny M, Balakrishnan G, Malik GM, Ghaly RF (1993) Cerebral oxygen metabolism during hypothermic circulatory arrest in humans. J Neurosurg 79: 810–815
Bickler PE, Litt L, Banville DL, Severinghaus J (1988) Effects of acetazolamide on cerebral acid-base balance. J Appl Physiol 65: 422–427
Brazy JE, Lewis DV, Mitnik MH, Jöbsis FF (1985) Noninvasive monitoring of cerebral oxygenation in preterm infants: preliminary observations. Pediatrics 75: 217–225
Bruhn H, Kleinschmidt A, Boecker H, Merboldt KD, Hanicke W, Frahm J (1994) The effect of acetazolamide on regional cerebral blood oxygenation at rest and under stimulation as assessed by MRI. J Cereb Blood Flow Metab 14: 742–748
Chan KH, Miller JD, Dearden NM, Andrews PJ, Midgley S (1992) The effect of changes in cerebral perfusion pressure upon middle cerebral artery blood flow velocity and jugular bulb venous oxygen saturation after severe brain injury. J Neurosurg 77: 55–61
Chance B, Leigh JS, Miyake H, Smith DS, Nioka S, Greenfield R, Einander M, Kaufmann K, Levy W, Young M (1988) Comparison of time-resolved and unresolved measurements of deoxygenation in brain. Proc Natl Acad Sci USA 85: 4971–4975
Cold GE, Christensen KJS, Nordentoft J, Engberg M, Bach P (1988) Cerebral blood flow, cerebral metabolic rate of oxygen and relative CO2 reactivity during neurolept anesthesia in patients subjected to craniotomy for supratentorial cerebral tumors. Acta Anesthesiol Scand 32: 310–315
Cope M, Delpy DT, Reynolds EOR, Wray S, Wyatt J, van der Zee P (1988) Methods of quantitating cerebral near infrared spectroscopy data. Adv Exp Med Biol 222: 183–189
Cruz J, Miner ME, Allen SJ, Alves WM, Gennarelli T (1990) Continuous monitoring of cerebral oxygenation in acute brain injury: injection of mannitol during hyperventilation. J Neurosurg 73: 725–730
Cruz J, Raps EC, Hoffstad OJ, Jaggi JL, Gennarelli TA (1993) Cerebral oxygenation monitoring. Crit Care Med 21: 1242–1246
Dearden NM (1991) Jugular bulb venous oxygen saturation in the management of severe head injury. Curr Opinion Anaesthesiol 4: 279–286
Delpy DT, Cope M, van der Zee P, Arridge S, Wray S, Wyatt S (1988) Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol 33: 1433–1442
Eggert HR, Blazek V (1987) Optical properties of human brain tissue, meninges and brain tumors in the spectral range of 200 to 900 nm. Neurosurgery 21: 459–464
Hans P, Franssen C, Damas F, Born JD, Lamy M (1991) Continuous measurement of jugular venous bulb oxygen saturation in neurosurgical patients. Acta Anaesth Belg 42: 213–218
Harris DNF, Bailey SM (1993) Near infrared spectroscopy in adults: does the INVOS 3100 really measure intracerebral oxygenation? Anaesthesia 48: 694–696
Hazeki O, Tamura M (1988) Quantitative analysis of hemoglobin oxygenation state of rat brain in situ by near-infrared spectrophotometry. J Appl Physiol 64: 796–802
Inoue Y, Momose T, Machida K, Honda N, Nishikawa J, Sasaki Y (1994) SPECT measurements of cerebral blood volume before and after acetazolamide in occlusive cerebrovascular disease. Radiat Med 12: 225–229
Jöbsis FF (1977) Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198: 1264–1267
Jöbsis FF, Keizer JH, LaManna JC, Rosenthal M (1977) Reflectance spectrophotometry of cytochrome aa3 in vivo. J Appl Physiol 43: 858–872
Kamei A, Ozaki T, Takashima S (1994) Monitoring of the intracranial hemodynamics and oxygenation during and after hyperventilation in newborn rabbits with near-infrared spectroscopy. Pediatr Res 35: 334–338
Kaminogo M, Ichikura A, Shibata S, Toba T, Yonekura M (1995) Effect of acetazolamide on regional cerebral oxygen saturation and regional cerebral blood flow. Stroke 26: 2358–2360
Kurth CD, Steven JM, Benaron D, Chance B (1993) Near-infrared monitoring of the cerebral circulation. J Clin Monit 9: 163–170
Leinsinger G, Schmiedek P, Kreisig T, Einhäupl K, Bauer W, Moser E (1988) 133Xe-DSPECT: significance of the cerebrovascular reserve capacity for the diagnosis and treatment of chronic cerebral ischaemia. Nucl Med 27: 127–134
Leinsinger G, Piepgras A, Einhäupl K, Schmiedeck P, Kirsch CM (1994) Normal values of cerebrovascular reserve capacity after stimulation with acetazolamide measured by xenon133 single-photon emission CT. Am J Neuroradiol 15: 1327–1332
McCormick PW, Stewart M, Lewis G (1991) Noninvasive measurement of regional cerebrovascular oxygen saturation in humans using optical spectroscopy. Proceedings SPIE-The International Society of Optical Engineering, Vol 1431, pp 294–302
McCormick PW, Stewart M, Goetting MG, Balakrishnan G (1991) Regional cerebrovascular oxygen saturation measured by optical spectroscopy in humans. Stroke 22: 596–602
McCormick PW, Stewart M, Goetting MG, Dujovny M, Lewis G, Ausman JI (1991) Noninvasive cerebral optical spectroscopy for monitoring cerebral oxygen delivery and hemodynamics. Crit Care Med 19: 89–97
Müller M, Voges M, Piepgras U, Schimrigk K (1995) Assessment of cerebral vasomotor reactivity by transcranial Doppler ultrasound and breath-holding. A comparison with acetazolamide as vasodilatory stimulus. Stroke 26: 96–100
Obrist WD, Thompson HK, Wang HS, Wilkinson WE (1975) Regional cerebral blood flow estimated by133Xenon inhalation. Stroke 6: 245–256
Piepgras A, Gückel F, Lämmler B, Weigel R, Schmiedeck P (1994) Non-invasive diagnosis in cerebral ischemia by means of magnetic resonance imaging and near-infrared spectroscopy. Radiologe 34: 627–631
Ringelstein EB, Van Eyck S, Mertens I (1992) Evaluation of cerebral vasomotor reactivity by various vasodilating stimuli: comparison of CO2 to acetazolamide. J Cereb Blood Flow Metab 12: 162–168
Robertson CS, Narayan RK, Gokaslan ZL, Pahwa R, Grossmann RG, Caram P, Allen E (1989) Cerebral arteriovenous oxygen difference as an estimate of cerebral blood flow in comatose patients. J Neurosurg 70: 222–230
Schroeder T (1986) Cerebrovascular reactivity to acetazolamide in carotid artery disease. Neurol Res 8: 231–236
Smielewski P, Kirkpatrick P, Minhas P, Pickard JD, Czonyka M (1995) Can cerebrovascular reactivity be measured with near-infrared spectroscopy? Stroke 26: 2285–2292
Sorteberg W, Lindegaard KF, Rootwelt K, Dahl A, Nyberg-Hansen R, Russel D, Nornes H (1989) Effect of acetazolamide on cerebral artery blood velocity and regional cerebral blood flow in normal subjects. Acta Neurochir (Wien) 97: 139–145
Vorstrup S, Henriksen L, Paulson OB (1984) Effect of acetazolamide on cerebral blood flow and cerebral metabolic rate for oxygen. J Clin Invest 74: 1634–1639
Vorstrup S, Brun B, Lassen NA (1986) Evaluation of the cerebral vasodilatory capacity by the acetazolamide test before ECIC bypass surgery in patients with occlusion of the internal carotid artery. Stroke 17: 1291–1298
Webster MW, Makaroun MS, Steed DL, Smith HA, Johnson DW, Yonas H (1995) Compromised cerebral blood flow reactivity is a predictor of stroke in patients with symptomatic carotid artery occlusive disease. J Vasc Surg 21: 338–344
Wray S, Cope M, Delpy DT, Wyatt JS, Reynolds EOR (1988) Characterization of the near infrared absorption spectra of cytochrome aa3 and hemoglobin for the non-invasive monitoring of cerebral oxygenation. Biochem Biophys Acta 933: 184–192
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Holzschuh, M., Woertgen, C., Metz, C. et al. Comparison of changes in cerebral blood flow and cerebral oxygen saturation measured by near infrared spectroscopy (NIRS) after acetazolamide. Acta neurochir 139, 58–62 (1997). https://doi.org/10.1007/BF01850869
Issue Date:
DOI: https://doi.org/10.1007/BF01850869