Summary
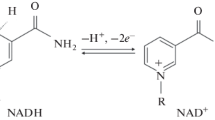
3-Aminopyridine mononucleotide, a nicotinamide mononucleotide analog, was prepared by enzymatic cleavage of 3-aminopyridine adenine dinucleotide by a snake venom phosphodiesterase and isolated by means of ion exchange chromatography. The spectrophotometric and fluorometric properties of this analog were studied. Several anions were shown to quench the fluorescence intensity of this analog. pH was shown to have a pronounced effect on the fluorescence intensity. 3-Aminopyridine mononucleotide was shown to be a coenzyme-competitive inhibitor of yeast alcohol dehydrogenase. The 3-aminopyridine mononucleotide was diazotized with the use of nitrous acid. A time dependent irreversible inactivation of yeast alcohol dehydrogenase resulted from incubation with the diazotized 3-aminopyridine mononucleotide at pH 7.0. Incubation of the enzyme with NAD prior to the addition of the diazotized 3-aminopyridine mononucleotide protected the enzyme against inactivation.
Recently, 3-aminopyridine adenine dinucleotide (AAD) and 3-aminopyridine adenine dinucleotide phosphate (AADP), NAD and NADP analogs respectively, were synthesized by either chemical or enzymatic processes. The chemical, spectrophotometric properties of these dinucleotides have also been reported. It was demonstrated that these nucleotides serve as coenzyme-competitive inhibitors of dehydrogenases but did not function as coenzymes for oxidation-reduction reactions catalyzed by these enzymes. The pyridine amino group of AAD was diazotized and the diazotized derivative was shown to inactive yeast alcohol dehydrogenase irreversibly. Isolation of modified cysteine residue from the modified yeast alcohol dehydrogenase resulting from inactivation by diazotized AAD has been reported. Thus, diazotized AAD proved to be a site specific label for the coenzyme binding site of yeast alcohol dehydrogenase. It was of interest to prepared and determine the properties of a NMN analog, 3-aminopyridine mononucleotide (APMN). The preparation of APMN was accomplished by enzymatic cleavage of AAD with snake venom phosphodiesterase according to a method previously reported. This report deals with the preparation, properties and studies of APMN with yeast alcohol dehydrogenase.
Similar content being viewed by others
References
Fisher, T. L., Vercellotti, S. V. and Anderson, B. M., 1973. J. Biol. Chem. 248, 4293–4299.
Anderson, B. M., Yuan, J. H. and Vercellotti, S. V., 1975. Mol. Cellular Biochem. 8, 89–96.
Chan, J. K. and Anderson, B. M., 1975. J. Biol. Chem. 250, 67–72.
Kaplan, N. O. and Stolzenbach, F. E., 1957. Methods in Enzymology, Colowick, S. P. and Kaplan, N. O. (editors), volume III, pp. 899–901, Academic Press, New York and London.
Lineweaver, H. and Burk, D. J., 1934. J. Amer. Chem. Soc. 56, 658–666.
Dixon, M., 1953. Biochem. J. 55, 170–171.
Kosower, E. M., 1962. Molecular Biochemistry, p. 180, McGraw-Hill Book Co., New York.
Weisstuch, A. and Testa, A. C., 1968. J. Phys. Chem. 72, 1982–1987.
Schofield, K., 1967. Hetro-Aromatic Nitrogen Compounds-Pyrroles and Pyridines, pp. 146–149, Butterworths, London.
Fiske, C. H. and Subbarow, Y., 1925. J. Biol. Chem. 66, 375–400.
Wagner-Jaurreg. T. and Moeller, E. F., 1935. Z. Physiol. Chem. 236, 222–227.
Taylor, J. F., Velick, S. F., Cori, G. T., Cori, G. F. and Slein, M. W., 1948. J. Biol. Chem. 173, 619–626.
Schales, O. and Schales, S. S., 1940. J. Biol. Chem. 140, 879–884.
Author information
Authors and Affiliations
Additional information
This work was supported in part by Research Grant GR-IX from Old Dominion University Research Foundation.
Rights and permissions
About this article
Cite this article
Woolf, J.H., Yuan, J.H. Studies of yeast alcohol dehydrogenase with 3-aminopyridine mononucleotide. Mol Cell Biochem 15, 19–25 (1977). https://doi.org/10.1007/BF01731286
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01731286