Summary
Brain slices are capable of a sustained formation of ammonia in vitro at a rate of 8–12 ßmoles/g wet wt/h during the first hour of incubation at 37°C. The reaction is aerobic and proceeds only in the absence of glucose or other easily assimilated carbohydrates.
In this article the questions of the origin of the ammonia formed and of the mechanism of its formation are discussed. Evidence is presented suggesting that ammonia is derived from free amino acids, either present initially or released by proteolysis during incubation. Glutamate and γ-aminobutyric acid (GABA) together account for about 50% of the ammonia formed. When brain slices are incubated in the presence of glucose, proteolysis appears to be somewhat reduced but a considerable increase in the level of glutamine indicates a continuation of ammonia formation followed by ammonia disposal in reactions leading to the synthesis of glutamine.
Amino acids added to the incubation medium cannot be utilized by brain slices as sources of ammonia. It is suggested that the endogenous amino acids which serve as ammonia precursors occur in a compartment not readily accessible to extracellular amino acids. This compartment probably consists largely of neurons.
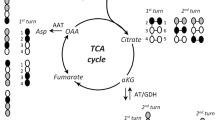
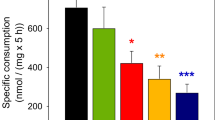
Glutamic acid is metabolized in brain either by transamination or by oxidative deamination. It is assumed that the choice between these two pathways is regulated mainly by the level of α-oxoglutarate and by the redox state of the mitochondrial NAD couple. A lowering of the steady state level of α-oxoglutarate, such as may be expected in the absence of glucose, would result in the oxidative deamination of glutamate. A significant role of glutamate dehydrogenase in the ammonia formation of brain slices is shown by the fact that a series of inhibitors of glutamate dehydrogenase also have an inhibitory effect on the ammonia formation of brain slices. Hadacidin, a specific inhibitor of adenylosuccinate synthetase, was also found to inhibit the ammonia formation of brain slices but to be only about half as efficient as inhibitors of glutamate dehydrogenase. The effects of hadacidin and 5-bromofuroate, an inhibitor of glutamate dehydrogenase, are additive. This is interpreted as indicating a minor involvement of the purine nucleotide cycle in the mechanism of ammonia release.
Similar content being viewed by others
References
H. Weil-Malherbe, in Neurochemistry (2nd. ed., K. A. C. Elliott, I. H. Page, and J. H. Quastel, editors) pp 321–330, C. C. Thomas, Springfield, Illinois (1962).
R. Vrba, Rev. Czechosl. Med. 3, 1–26 (1957).
Y. Tsukada, in Handbook of Neurochemistry (A. Lajtha, editor) Vol. V, pp 215–233, Plenum Press, New York (1971).
D. H. Labby, J. H. Hutchinson, J. Stahl, R. Bockel and M. Imler, in Biochemical Clinics, The Liver, 4, 245, R. H. Donnelley Corp., New York (1964).
R. T. Manning, in Biochemical Clinics, The Liver, 4, 225, R. H. Donnelley Corp., New York (1964).
S. Schenker, D. W. McCandles, E. Brophy and M. S. Lewis, J. clin. Invest. 46, 838–848 (1967).
O. Warburg, K. Posner and E. Negelein, Biochem. Z. 152, 309–344 (1924).
F. Dickens and G. D. Greville, Biochem. J. 27, 1123–1133 (1933).
R. O. Loebel, Biochem. Z. 161, 219–239 (1925).
H. Weil-Malherbe and R. H. Green, Biochem. J. 61, 210–218 (1955).
H. Weil-Malherbe and J. Gordon, J. Neurochem. 18, 1659–1672 (1971).
C. Riebeling, Klin. Woch. 10, 554 (1931).
H. Schwarz and H. Dibold, Biochem. Z. 251, 190–198 (1932).
S. R. Guha and J. J. Ghosh, Ann. Biochem. Exp. Med. 19, 67–74 (1959).
S. R. Guha, B. N. Ghosh and J. J. Ghosh, Ann. Biochem. Exp. Med. 19, 255–264 (1959).
G. Takagaki, S. Hirano and Y. Tsukada, Arch. Biochem. Biophys. 68, 196–205 (1957).
M. G. Larrabee, P. Horowicz, W. Stekiel and M. Dolivo, in Metabolism of the Nervous System (D. Richter, editor) pp 208–220, Pergamon Press, London (1957).
H. Waelsch, Lancet II, 1–4 (1949).
H. A. Krebs, L. V. Eggleston and R. Hems, Biochem. J. 44, 159–163 (1949).
H. A. Krebs, Biochem. J. 29, 1951–1969 (1935).
H. Weil-Malherbe, Biochem. J. 30, 665–676 (1936).
H. Weil-Malherbe and A. C. Drysdale, J. Neurochem. 1, 250–255 (1957).
Y. Tsukada and G. Takagaki, Nature 173, 1138–1139 (1954).
G. Acs, R. Balázs and F. B. Straub, Chem. Abstr. 48, 8923 (1954).
R. Vrba, J. Neurochem. 1, 12–17 (1956).
Y. Yoshino and K. A. C. Elliott, Can. J. Biochem. 48, 1175–1178 (1970).
Y. Yoshino and K. A. C. Elliott, Can. J. Biochem. 48, 236–243 (1970).
R. Vrba, J. Folbergr and V. Kanturek, Nature 179, 470–471 (1957).
P. A. Kometiani, Ye. E. Klein, N. V. Gvalia and Ye. G. Gotsiridze, J. Neurochem. 17, 1331–1337 (1970).
G. B. Ansell, R. B. Williams and D. Richter, Biochem. J. 50, XXIX (1952).
E. L. Peters and D. B. Tower, J. Neurochem. 5, 80–90 (1959).
H. Waelsch, in Regional Neurochemistry (S. S. Kety, and J. Elkes, editors) pp 57–64, Pergamon Press, Oxford (1961).
G. Takagaki, S. Berl, D. D. Clarke, D. P. Purpura and H. Waelsch, Nature 189, 326 (1961).
S. Berl, G. Takagaki, D. D. Clarke and H. Waelsch, J. biol. Chem. 237, 2562–2569 (1962).
R. M. O'Neal and R. E. Koeppe, J. Neurochem. 13, 835–847 (1966).
J. E. Cremer, J. Neurochem. 11, 165–185 (1964).
D. Garfinkel, J. biol. Chem. 241, 3918–3929 (1966).
K. Okamoto and J. H. Quastel, Biochem. J. 128, 1117–1124 (1972).
F. Hajós and S. Kerpel-Fronius, J. Cell. Biol. 51, 216–222 (1971).
R. Balázs, A. J. Patel and D. Richter, in Metabolic Compartmentation in the Brain (R. Balázs and J. E. Cremer, editors) pp 167–184, John Wiley and Sons, New York (1972).
S. P. R. Rose, in Metabolic Compartmentation in the Brain (R. Balázs and J. E. Cremer, editors) pp 287–304, John Wiley and Sons, New York (1972).
A. Hamberger, Brain Res. 31, 169–178 (1971).
S. P. R. Rose, J. Neurochem. 17, 809–816 (1970).
A. E. Braunstein, Advances in Enzymology, 19, 335–389 (1957).
P. Borst, Biochim. biophys. Acta 57, 256–269 (1962).
E. A. Jones and H. Gutfreund, Biochem. J. 84, 46–51. (1962)
E. J. DeHaan, J. M. Tager and E. C. Slater (1967) Biochim. biophys. Acta, 131, 1–13 (1967).
H. A. Krebs and D. Bellamy, Biochem. J. 75, 523–529. (1960).
R. J. Haslam and H. A. Krebs, Biochem. J. 88, 566–578 (1963).
H. v. Euler, E. Adler, G. Günther and N. B. Das, Z. physiol. Chem. 254, 61–103 (1938).
H. J. Strecker, Arch. Biochem. Biophys. 46, 128–140 (1953).
P. C. Engel and K. Dalziel, Biochem. J. 105, 691–695 (1967).
R. Vrba, M. K. Gaitonde and D. Richter, J. Neurochem. 9, 465–475 (1962).
R. Balázs and R. J. Haslam, Biochem. J. 94, 131–141 (1965).
O. Gonda and J. H. Quastel, Biochem. J. 84, 394–406 (1962).
A. L. Miller, R. A. Hawkins and R. L. Veech, J. Neurochem. 20, 1393–1400 (1973).
D. H. Williamson, P. Lund and H. A. Krebs, Biochem. J. 103, 514–527 (1967).
D. Veloso, J. V. Passonneau and R. L. Veech, J. Neurochem. 19, 2679–2686 (1972).
R. L. Veech, R. L. Harris, D. Veloso and E. H. Veech, J. Neurochem. 20, 183–188 (1973).
A. L. Miller, R. A. Hawkins and R. L. Veech, In preparation.
H. Weil-Malherbe, Unpublished.
W. S. Caughey, J. D. Smiley and L. Hellerman, J. biol. Chem. 224, 591–607 (1957).
K. S. Rogers, J. biol. Chem. 246, 2004–2009 (1971).
G. Takagaki, S. Hirano and Y. Nagata, J. Neurochem. 4, 124–134 (1959).
H. Weil-Malherbe and J. Gordon, Neuropharmacol. 12, 367–381 (1973).
A. J. Garber, M. Jomain-Baum, L. Salganicoff, E. Farber and R. W. Hanson, J. biol. Chem. 248, 1530–1535 (1973).
P. A. Kometiani and E. E. Klein, Biokhimüa, 21, 389 (1956).
R. Abrams and M. Bentley, Arch. Biochem. Biophys. 58, 109–118 (1955).
C. E. Carter and L. H. Cohen, J. biol. Chem. 222, 17–30 (1956).
I. Lieberman, J. biol. Chem. 223, 327–339 (1956).
A. A. Newton and S. V. Perry, Biochem. J. 74, 127–136 (1960).
C. L. Davey, Arch. Biochem. Biophys. 95, 296–304 (1961)
K. Tornheim and J. M. Lowenstein, J. biol. Chem. 247, 162–169 (1972).
J. M. Lowenstein, Physiol. Rev. 52, 382–414 (1972).
H. C. Buniatian, in Handbook of Neurochemistry (A. Lajtha, editor) Vol. III, pp 399–413, Plenum Press, New York (1970).
J. Abelskov, Biochim. biophys. Acta, 32, 566 (1959).
H. T. Shigeura and C. N. Gordon, J. biol. Chem. 237, 1932–1936 (1962).
S. Berl, S. Puszkin and W. J. Nicklas, Science, 179, 441–446 (1973).
Author information
Authors and Affiliations
Additional information
An invited article.
Rights and permissions
About this article
Cite this article
Weil-Malherbe, H. Ammonia formation in brain slices. Mol Cell Biochem 4, 31–44 (1974). https://doi.org/10.1007/BF01731101
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01731101