Abstract
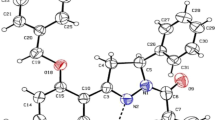
The structure and conformation of (1)-Centbutindole, a newly marketed neuroleptic compound, has been investigated by X-ray crystallography. It crystallizes in the monoclinic system and the non-centrosymmetric space group P21 (Z=2) with cell dimensionsa=8.434(6),b=6.620(3),c=18.419(9)Å, and β=95.07(6)°. The chain conformation istrans extended. The embedded Δ3 piperidine ring exists in ahalf-chair conformation whereas the embedded piperazine ring exists in achair conformation. The propylene side chain is equatorial relative to the piperazine. A systematic conformational analysis of centbutindole and of a related molecule, Haloperidol, followed by a Monte Carlo search and a stochastic dynamics simulation, have been performed. Electronic and lipophilic properties have been computed and, molecular electrostatic potential (MEP) maps and molecular lipophilicity potential (MLP) maps, have been displayed for two selected conformers satisfying a reported pharmacophore. The two molecules exhibit very similar molecular properties.
Similar content being viewed by others
References
Saxena, A. K.; Jain, P. C.; Anand, N.; Dua, P. R.J. Med. Chem. 1973,16, 560.
Saxena, A. F.; Jain, P. C.; Anand, N.; Dua, P. R.Drugs of the Future; Prous, J. R., Ed.; Barcelona,1978, Vol. 3, p. 803.
Kumar, N.; Dhaon, M. K.; Agarwal, S. K.; Saxena, A. K.; Jain, P. C.; Prasad, C. R.; Anand, N.Eur. J. Med. Chem. 1982,17, 312.
Jain, P. C.; Saxena, A. K.; Kumar, N.; Dhaon, M. K.; Agarwal, S. K.; Anand, N.; Raghubir, R.; Singh, H. K.; Srimal, R. C.; Dhawan, B. N.Ind. J. Pharm. 1976,38, 150.
Seeman, P.; Westman, K.; Protiva, M.; Lilek, J.; Jain, P. C.; Saxena, A. K.; Anand, N.; Humber, L.; Philipp, A.Eur. J. Pharmacol. 1979,56, 247.
Dhawan, B. N.; Dua, P. R.; Gupta, P. P.; Raghubir, R.; Singh, H. K., Saxena, A. K.; Anand, N.Pharmacology for Health in Asia; Allied Publishers: New Dehli,1987; pp. 193–201.
Main, P.; Fiske, S. J.; Hull, S. E.; Lessinger, L.; Germain, G.; Declercq, J.-P.; Woolfson, M. M.Multan 80, A System of Computer Programs for the Automatic Solution of Crystal Structures from X-ray Diffraction Data; Univs. of York, U. K. and Louvain: Belgium,1980.
Mohamadi, F.; Richards, N. G. J.; Guida, W. C.; Liskamp, R.; Lipton, M.; Caufield, C.; Chang, G.; Hendrikson, T.; Still, W. C.J. Comp. Chem. 1990,11, 441.
Allinger, N. L.J. Am. Chem. Soc. 1977,99, 8127.
De Clercq, P. J.; Hoflack, J. M.A computer program for systematic conformational analysis: Univ. of Ghent: Belgium.
Chang, G.; Guida, W. C.; Still, W. C.J. Am. Chem. Soc. 1989,111, 4379.
Saunders, M.; Houk, K. N.; Wu, Y.-D.; Still, W. C.; Lipton, M.; Chang, G.; Guida, W. C.J. Am. Chem. Soc. 1990,112, 1419.
Van Gunsteren, W. F.; Berendsen, H. J. C.Molec. Simul. 1988,1, 173.
CHEM-X, developed and distributed by Chemical Design Ltd, Chipping Norton, U.K.
Chung-Philips, A.J. Comp. Chem. 1989,10, 17.
Giessner-Prettre, C.Quantum Chemistry Program Exchange 1974,11, 249.
Audry, E.; Dubost, J.-P.; Colleter, J.-C.; Dallet, Ph.Eur. J. Med. Chem. 1986,21, 71.
Fauchère, J.-L.; Quarendon, P.; Kaetterer, L.J. Mol. Graphics 1988,6, 203.
INSIGHT II, developed and distributed by Biosym Technologies, San Diego.
Broto, P.; Moreau, G.; Vandycke, C.Eur. J. Med. Chem. 1984,19, 71.
Croizet, F.; Dubost, J.-P.; Langlois, M.-H.; Audry, E.Quant. Struct.-Act. Relat. 1991,10, 211.
Reed, L. L.; Schaefer, J. P.;Acta Cryst. 1973, B29, 1886.
Datta, N.; Mondal, P.; Pauling, P.Acta. Cryst. 1979, B35, 1486.
Keller, C. E.; Karr, P. A.; Zandler, M. E.; Carper, W. R.Struct. Chem. 1992,3, 195.
Koch, M. H. J.; Germain, G.Acta. Cryst. 1972, B28, 12.
Koch, M. H. J.Acta. Cryst. 1973, B29, 379.
Declercq, J. P.; Germain, G.; Koch, M. H. J.Acta. Cryst. 1973, B29, 2311.
Koch, M. H. J.; Evrard, G.Acta. Cryst. 1973, B29, 2971.
Declercq, J. P.; Germain, G.; Koch, M. H. J.Acta. Cryst. 1975, B31, 628.
Michel, A. G.; Evrard, G.; Schiltz, M.; Durant, F.; Koch, M. H. J.Acta. Cryst. 1976, B32, 2507.
Van Opdenbosch, N.; Evrard, G.; Dorval, C.; Durant, F.; Koch, M. H. J.Acta. Cryst. 1977, B33, 171.
Shenkin, P. S.; McDonald, D. Q.J. Comput. Chem. 1994,15, 899.
Froimowitz, M.; Baldessarini, R. J.J. Pharm. Sci. 1987,76, 557.
Liljefors, T.; Bogeso, K. P.J. Med. Chem. 1988,31, 306.
Rognan, D.; Sokoloff, P.; Mann, A.; Martres, M.-P.; Schwartz, J.-C.; Costentin, J.; Wermuth, C.-G.,Eur. J. Pharmacol. 1990,189, 59.
Bogeso, K. P.; Liljefors, T.; Arnt, J.; Hyttel, J.; Pedersen, H.J. Med. Chem. 1991,34, 2023.
Froimowitz, M.; Rämsby, S.J. Med. Chem. 1991,34, 1707.
De Paulis, T.; El Tayar, N.; Carrupt, P.-A.; Testa, B.; Van de Waterbeemd, H.Helv. Chim. Acta. 1991,74, 241.
Pettersson, I.; Liljefors, T.J. Med. Chem. 1992,35, 2355.
Froimowitz, M.; Cody, V.J. Med. Chem. 1993,36, 2219.
Palm, J.; Bogeso, K. P.; Liljefors, T.J. Med. Chem. 1993,36, 2878.
Collin, S.; Evrard, G.; Vercauteren, D. P.; Durant, F.; Carrupt, P.-A.; Van de Waterbeemd, H.; Testa, B.J. Med. Chem. 1989,32, 38.
Rusig, I.; Laguerre, M.; Carpy, A.; Saxena, A. K.Med. Chem. Res. 1995, Submitted.
Desjarlais, R. L.; Seibel, G. L.; Kuntz, I. D.; Furth, P. S.; Alvarez, J. C.; Ortiz De Montellano, P. R.; DeCamp, D. L.; Babe, L. M.; Craik, C. S.Proc. Natl. Acad. Sci. 1990,87, 6644.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rusig, I., Léger, J.M., Laguerre, M. et al. Structure and molecular properties of (1)-Centbutindole. Comparison with Haloperidol. J Chem Crystallogr 25, 443–451 (1995). https://doi.org/10.1007/BF01665699
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01665699