Abstract
A prospective, randomized, controlled study of adjuvant chemotherapy in operable gastric cancer was commenced in 1976. Four hundred and eleven patients have been randomized into 1 of 3 treatment groups. Group A received a placebo injection of intravenous normal saline (10 ml) at 3-week intervals. Group B received a 5-day induction course of 5-fluorouracil (5-FU), vincristine, cyclophosphamide, and methotrexate followed by 3-week intravenous injections of 5-FU and mitomycin C (MMC). Group C received 3-week intravenous injections of 5-FU and MMC. The treatment was to be given for 2 years. Patients were stratified for age, sex, preoperative duration of symptoms, and clinicopathological stage prior to randomization. At this interim analysis, preoperative duration of symptoms and clinicopathological stage were statistically significant prognostic factors (p=<0.05 and <0.001, respectively).
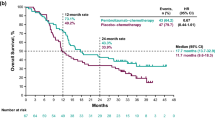
At the time of this analysis, there has been no significant improvement in survival in either of the treatment groups compared to the control group. While treatment could begin at any time during the first 3 postoperative months, survival at 1 year was significantly improved (p=0.013) by treatment C in those who started treatment within 1 month of surgery; however, this benefit of treatment was not maintained at 3 years. Of the 279 deaths, 16 (5.7%) have been drug-related, 11 resulting from renal toxicity associated with this therapeutic regimen.
This study has confirmed the poor prognosis of gastric cancer, and the early results demonstrate that there is no place for the use of cytotoxic agents in this disease outside prospective controlled studies.
frRésumé
Une étude prospective organisée et contrÔlée de la chimiothérapie complémentaire de la chirurgie du cancer de l'estomac opéré a commencé en 1976.
411 malades ont été répartis en trois groupes. Les opérés du groupe A ont reÇu un placébo, c'est-àdire une injection intraveineuse de 10 ml de sérum salé isotonique à 3 semaines d'intervalle. Ceux du groupe B ont reÇu d'abord durant 5 jours du 5-fluorouracil (5FU), de la vincristine, de la cyclophosphaline et du methotrexate puis toutes les trois semaines des injections de 5FU et de mitomycine (MMC). Enfin ceux du groupe C ont reÇu toutes les semaines des injections de 5FU et de MMC. Le traitement a duré deux ans.
Avant la randomisation, les patients ont été répartis selon l'âge, le sexe, la durée des symptÔmes, le stade anatomoclinique. L'analyse provisoire a permis de constater que la durée des symptÔmes avant l'intervention et que le stade anatomo-clinique atteint par le cancer étaient des facteurs de pronostic statistiquement significatifs (P=<0.05 et <001 rétrospectivement). Au terme de l'analyse, on peut cependant affirmer qu'il n'y a pas d'amélioration du taux de la survie des malades traités par rapport à ceux qui reÇurent un placebo. S'il est à remarquer que la survie à un an a été significativement améliorée (P=0,013) quand le traitement de type C a débuté au plus tard un mois avant l'intervention, le bénéfice de la chimiothérapie ne se maintient pas à trois ans. Enfin, sur les 279 morts dénombrés, 16 (5,7%) peuvent Être attribués à l'emploi des agents cytotoxiques, 11 de ces morts relevant de la toxicité pour le rein de ces drogues.
Cette étude confirme le pronostic sévère du cancer de l'estomac et les résultats démontrent qu'il n'y a réellement pas de place pour l'emploi d'agents cytotoxiques dans le traitement du cancer de l'estomac.
Abstracto
En 1976 se inició un estudio prospectivo, al azar y controlado de la quimioterapia coadyuvante en cáncer gástrico operable. Cuatrocientos once patientes fueron asignados al azar a 1 de 3 grupos de tratamiento. El grupo A recibió placebo en forma de una inyección intravenosa de solución salina normal (10 ml) a intervalos trisemanales. El grupo B recibió un curso de 5 días de inducción de 5-fluoruracilo (5-FU), vincristina, ciclofosfamida y metotrexato seguido de inyecciones de 5-FU en inyecciones trisemanales de 5-FU y mitomicina C (MMC). El grupo C recibió inyecciones trisemanales de 5-FU y MMC. El tratamiento ha sido proyectado para 2 años. Los pacientes fueron estratificados por edad, sexo, duración preoperatoria de los síntomas, y estado clínico patológico anterior a la asignación al azar. En el momento de este análisis preliminar, la duraćion preoperatoria de los síntomas y el estado clínicopatologico aparecen como factores de pronóstico de significaćion estadística (p=<0.05 y <0.001, respectivamente).
En la fecha de este análisis no se ha observado ninguna mejoría significativa en la supervivencia en ninguno de los grupos de tratamiento comparados con el grupo control. Aun cuando el tratamiento puede iniciarse en cualquier momento en el curso de los primeros 3 meses, la supervivencia a 1 año fue significativamente mejor (p=0.013) con el tratamiento C en aquellos pacientes que comenzaron tratamiento dentro del mes siguiente a la cirugía; sin embargo, este beneficio del tratamiento no fue mantenido a los 3 años. De las 279 muertes, 16 (5.7%) han estado relacionadas con las drogas, 11 como resultado de toxicidad renal asociada con este regimen terapéutico.
Este estudio ha confirmado el pobre pronóstico del cáncer gástrico, y los resultados preliminares demuestran que no existe lugar para el uso de agentes citotóxicos por fuera de estudios prospectivos controlados, en esta enfermedad.
Similar content being viewed by others
References
1979 Mortality statistics—cause. England and Wales. Office of Population Census and Surveys. Malignancies and neoplasms of the stomach, p. 10
Waterhouse, J.A.H.: Cancer Handbook of Epidemiology and Prognosis. Edinburgh, Churchill Livingstone, 1974
Swynnerton, R.F., Truelove, S.C.: Carcinoma of the stomach. Br. Med. J.1:287, 1952
Takagi, K.: Stages of gastric cancer and reconstruction after surgery. In Gastric Cancer: Advances in the Biosciences, vol. 32, J.W.L. Fielding, C.E. Newman, C.H.J. Ford, B.G. Jones, editors, Oxford, Pergamon Press, 1981, pp. 191–202
Fielding, J.W.L., Ellis, D.J., Jones, B.G., Paterson, J., Powell, D.J., Waterhouse, J.A., Brookes, V.S.: Natural history of “early” gastric cancer: Results of a 10-year regional survey. Br. Med. J.281:965, 1980
Gunderson, L.: Radiation therapy: Results and future possibilities. Clin. Gastroenterol.5:743, 1976
Kajitani, T., Miwa, K.: Treatment results of stomach carcinoma in Japan 1963–1966. WHO-CC Monograph 2, Tokyo, WHO, 1979
Gilbertson, V.A.: Results of treatment of stomach cancer. Cancer23:1305, 1969
Comis, R., Carter, S.: A review of chemotherapy in gastric cancer. Cancer34:1576, 1974
Rake, M.O., Mallinson, C.N., Cocking, B.J., Cwynarski, M.C., Fox, C., Jackson, A., Diffey, B.: Assessment of the value of cytotoxic therapy in treatment of carcinoma of the stomach. Gut17:832, 1976
Peto, R., Pike, M.C., Armitage, P., Breslow, N.E., Cox, D.R., Howard, S.V., Mantel, N., McPherson, K., Peto, J., Smith, P.G.: Design and analysis of randomised clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br. J. Cancer35:1, 1977
Brookes, V.S., Waterhouse, J.A.H., Powell, J.: Carcinoma of the stomach: 10-year survey of results and of factors affecting prognosis. Br. Med. J.1:1577, 1965
Smith, F.P., Cambareri, R.J., Killen, J.Y., et al.: Gastrointestinal cancer. In Cancer Chemotherapy: The EORTC Cancer Chemotherapy Annual 2, H. Pindeo, editor, Amsterdam, Excerpta Medica, 1980, pp. 284–298
Kovach, J.S., Moertel, C.G., Schutt, A.J., Hahn, R.G., Reitemeier, R.J.: A controlled study of 1, 3, BIS-(2 chloroethyl)-1-nitrosourea and 5-fluorouracil therapy for advanced gastric cancer and pancreatic cancer. Cancer33:563, 1974
Reitemeier, R.J., Moertel, C.G., Hahn, R.G.: Combination chemotherapy in gastrointestinal cancer. Cancer Res.30:1425, 1970
Hanham, I., Newton, K., Westbury, G.: 75 cases of solid tumours treated by a modified quadruple chemotherapy regime. Br. J. Cancer25:462, 1971
Kingston, R.D., Ellis, D.J., Powell, J., Brookes, V.S., Waterhouse, J.A., Hurst, M.D., Smith, J.A.: The West Midlands gastric carcinoma chemotherapy trial: Planning and results. Clin. Oncol.4:55, 1978
Imanaga, H., Nakazoto, H.: Results of surgery for gastric cancer and the effect of adjuvant mitomycin C on cancer recurrence. World J. Surg.1:213, 1977
Franz, J.L., Cruz, A.B.: The treatment of gastric cancer with combined surgical dissection and chemotherapy. J. Surg. Oncol.9:131, 1977
Lundh, G., Burn, J.I., Kolig, G., et al.: A cooperative international study of gastric cancer. Ann. R. Coll. Surg. Engl.54:219, 1974
Jones, B.G., Fielding, J.W.L., Newman, C.E., Howell, A., Brookes, V.S.: Intravascular haemolysis and renal impairment after blood transfusion in two patients on long term 5-Fluorouracil and Mitomycin C. Lancet1:1275, 1980
Rumpf, K.W., Reiger, J., Lankisch, P.G., von Heyden, H.W., Nagel, G.A., Scheler, F.: Mitomycin C induced haemolysis and renal failure. Lancet2:1037, 1980
Jones, B.G. (for the Stomach Cancer Group): Design and progress of a multi-centre trial of adjuvant chemotherapy in operable gastric cancer. In Progress and Perspectives in the Treatment of Gastrointestinal Tumours, A. Gerard, editor, Oxford, Pergamon Press, 1981, pp. 36–40
Nakajima, T., Kajitani, T.: Surgical treatment of gastric cancer with special reference to lymph node dissection. In Diagnosis and Treatment of Upper Gastrointestinal Tumours, M. Friedman, M. Ogawa, D. Kisner, editors, Amsterdam, Excerpta Medica, 1981, pp. 207–223
The Gastrointestinal Tumour Study Group: Controlled trial of adjuvant chemotherapy following curative resection for gastric cancer. Cancer49:1116, 1982
Bitran, J.D., Desser, R.K., Kozloff, M.F., Billings, A.A., Shapiro, C.M.: Treatment of metastatic pancreatic and gastric adenocarcinomas with 5-Fluorouracil, Adriamycin and Mitomycin C. Cancer Treat. Rep.63:2049, 1979
Levi, J.A., Dalley, D.N., Aroney, R.S.: Improved combination chemotherapy in advanced gastric cancer. Br. Med. J.2:1471, 1979
Robinson, E., Cohen, Y.: The combination of surgery, radiotherapy and chemotherapy in the treatment of gastric cancer. Recent Results Cancer Res.32:177, 1977
Moertel, C.G., Childs, D.S., Jr., Reitemeier, R.J., Colby, M.Y., Jr., Holbrook, M.A.: Combined 5-Fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet2:865, 1969
Catterall, M., Kingsley, D., Lawrence, G., Grainger, J., Spencer, J.: The effects of fast neutrons on inoperable carcinoma of the stomach. Gut16:150, 1975
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fielding, J.W.L., Fagg, S.L., Jones, B.G. et al. An interim report of a prospective, randomized, controlled study of adjuvant chemotherapy in operable gastric cancer: British stomach cancer group. World J. Surg. 7, 390–399 (1983). https://doi.org/10.1007/BF01658089
Issue Date:
DOI: https://doi.org/10.1007/BF01658089