Summary
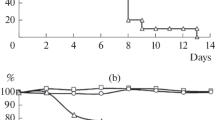
Two inactivated influenza-virus vaccines were tested and compared in three army training units in Israel. The serological responses to the vaccines and the side-effects were assessed. The vaccines contained the influenza strains which were prevalent in 1974: A2/Port Chalmers/1/73 and B/Hong Kong/8/73. One of the vaccines also contained A2/England/42/72. Both vaccines caused a more than three-fold rise in geometric mean titers against influenza A strains, and about a twofold rise in geometric mean titers against influenza B/Hong Kong/5/73. Approximately 75%–80% of the vaccinees acquired protective hemagglutination-inhibition antibody titers against influenza A strains, while less than 30% acquired protective titers against B strains. In general, there were no significant differences between the serological responses to the two vaccines. More than 50% of the vaccinees experienced at least one systemic side-effect (50.3% with one vaccine and 61.0% with the other). The average number of side-effects per person was between 1.78 and 2.11. However, these side-effects were generally of short duration and caused minimal disability. On the whole, the two vaccines did not differ significantly with regard to the side-effects they caused.
Zusammenfassung
Es wurden zwei inaktivierte Influenzavirus-Vakzine geprüft und bei drei Ausbildungseinheiten der Armee in Israel miteinander verglichen. Die serologischen Reaktionen auf die Vakzine und die Nebenwirkungen wurden ermittelt. Die Vakzine enthielten die 1974 weit verbreiteten Influenzastämme: A2/Port Chalmers/1/73 und B/Hongkong/8/73. Eine der Vakzine enthielt außerdem A2/England/42/72. Beide Vakzinen führten zu einem mehr als dreifachen Ansteigen der geometrischen mittleren Titer gegen die Influenza-A-Stämme und zu einem etwa zweifachen Ansteigen der geometrischen mittleren Titer gegen Influenza B/Hongkong/5/73. Etwa 75–80% der Vakzine bewirkten protektive Hämagglutinationshemmung-Antikörpertiter gegen Influenza-A-Stämme, während weniger als 30% Antikörpertiter gegen B-Stämme bewirkten. Hinsichtlich der serologischen Reaktionen bestanden zwischen den zwei Vakzinen im allgemeinen keine signifikanten Unterschiede. Mehr als 50% der Geimpften verspürten mindestens eine allgemeine Nebenwirkung (50,3% bei der einen Vakzine, und 61,0% bei der anderen). Die durchschnittliche Zahl der Nebenwirkungen pro Person lag zwischen 1,78 und 2,11. Diese Nebenwirkungen waren allerdings im allgemeinen von kurzer Dauer und lösten eine geringfügige Beeinträchtigung aus. Insgesamt betrachtet, unterschieden sich die zwei Vakzine hinsichtlich der hervorgerufenen Nebenwirkungen nicht wesentlich voneinander.
Similar content being viewed by others
Literature
Beare, A. S., Hobson, D., Redd, S. E., Tyrrell, D. A. J. A comparison of live and killed influenza virus vaccines. Lancet 2 (1968) 418–420.
Brown, P., Gajdusek, D. C., Chen, K. M., Morris, J. A. Antigenic response to influenza virus in man. II: Neutralizing antibody response to inactivated monovalent B vaccine, with observations on vaccine efficacy during a subsequent type B epidemic. Am. J. Epid. 90 (1969) 336–343.
Brown, B., Gajdusek, D. C., Morris, J. A. Antigenic response to influenza virus in man: I. Neutralizing antibody response to inactivated monovalent A 2 vaccine as related to prior influenza exposure. Am. J. Epid. 90 (1969) 327–335.
Peck, F. B. Purified influenza virus vaccine-a study of viral reactivity and antigenicity. J. Am. Med. Ass. 206 (1968) 2277–2282.
Mostow, S. R., Schoenbaum, S. C., Dowdle, W. R., Coleman, M. T., Kaye, H. S., Hierholzer, J. C. Studies on inactivated influenza vaccines. II. Effect of increasing dosage on antibody response and adverse reactions in man. Am. J. Epid. 92 (1970) 248–256.
Philips, C. A., Forsyth, B. R., Christmas, W. A., Gump, D. W., Worton, E. B., Rogers, I., Rudin, A. Purified influenza vaccines: Clinical and serological responses to varying doses and different routes of immunization. J. Inf. Dis. 122 (1970) 126–132.
Waldman, R. H., Coggins, W. J. Influenza immunization: Field trial on a university campus. J. Inf. Dis. 126 (1972) 242–248.
Howells, C. H. L., Evans, A. D., Vasselinova-Jenkins, C. Effect of two doses of influenza vaccine in stimulating antibody in volunteers. Lancet 1 (1973) 1436–1438.
Masurel, N., Anker, W. J. J., Nommensen, F. E. Efficacy of influenza vaccines. Lancet 2 (1974) 1067–1077.
Recommendations of the Public Health Service Advisory Committee on Immunization Practices-Influenza vaccine. C. D. C. Morbid. Mort. Weekly Report 24 (1975) 197–198.
Goyne, J. F. Influenza immunization and lost time in industry. J. Occup. Med. 11 (1969) 311–318.
Taylor, P. J., Miller, C. L., Pollock, T. M., Perkins, F. T., Westwood, M. A. Antibody response and reactions to aqueous influenza vaccine, simple emulsion vaccine and multiple emulsion vaccine. A report to the Medical Research Council Committee on influenza and other respiratory viruses. J. Hyg. Camb 67 (1969) 485–490.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Egoz, N., Morag, B., Klingberg, W. et al. Influenza immunization: Serologic and clinical responses in military units. Infection 5, 71–75 (1977). https://doi.org/10.1007/BF01642083
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01642083