Summary
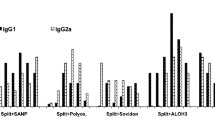
Our investigation indicates that pretreatment of human immunoglobulin for the elimination of the anticomplementary activity is associated with a loss of activity, the extent of which depends on the type of treatment applied. Laboratory preparations of human IgG were tested in a mouse protection assay using influenza A2-Taiwan virus, tetanus toxin andSalmonella typhimurium as the challenge. There was a 7–28% reduction in efficacy in an intravenous 7S preparation in comparison with an untreated 7S IgG. F(ab′)2 fragments showed a 24–65% and Fab fragments an 80–100% reduction in efficacy. Two commercial human 7S products showed approximately 90% efficacy in the Salmonella assay; a commercial, pepsin-treated preparation showed 65–74% efficacy when compared with untreated 7S IgG.
Zusammenfassung
Die hier dargelegten Untersuchungen zeigen, daß die Vorbehandlung humanen Immunglobulins zur Elimination der antikomplementären Aktivität mit einem Wirkungsverlust verbunden ist, dessen Ausmaß von der Art der Vorbehandlung abhängig ist. In Mäuseschutzversuchen wurde gefunden, daß die Wirksamkeit von Laborpräparationen humanen, i. v.-verträglichen Immunglobulins gegen Influenza A2-Taiwan Virus, Tetanustoxin undSalmonella typhimurium um ca. 7–28% bei 7S-Immunglobulin im Vergleich zu unbehandeltem Standard-IgG reduziert wurde. F(ab′)2-Fragmente zeigten 24–65%, Fab-Fragmente 80–100% Verlust der Wirksamkeit. Zwei käufliche, humane 7S IgG-Präparationen für intravenöse Anwendung zeigten im Salmonella-Assay ca. 90% Wirksamheit, ein käufliches, pepsin-behandeltes IgG-Präparat ca. 65–74% der Wirksamkeit eines unbehandelten 7S IgG-Präparates.
Similar content being viewed by others
Literature
Römer, J., Späth, P. J. Molecular composition of immunoglobulin preparations and its relation to complement activation. In:Nydegger, U. E. (ed.): Immunotherapy. Academic Press, London, New York 1981, pp. 123–130.
Skvaril, F., Barandun, S.: In vitro characterization of immunoglobulins for intravenous use. In:Alving, B. M., Finlayson, J. S. (eds.): Immunoglobulins: Characteristics and uses of intravenous preparations. DHHS Publication No. (FDA)-80-9005, Washington, D.C. 1980, pp. 201–206.
Barandun, S., Skvaril, F., Morell, A. Prophylaxe und Therapie mit γ-Globulin. Allgemeine Charakterisierung und klinische Anwendung von γ-Globulin-Präparaten. Schweiz. Med. Wochenschr. 106 (1976) 533–542, 580–586.
Cremer, N. E., Riggs, J. L., Lennette, E. H. Neutralization kinetics of Western equine encephalitis virus by antibody fragments. Immunochemistry 12 (1975) 597–601.
Römer, J., Morgenthaler, J.-J., Scherz, R., Skvaril, F. Characterization of various immunoglobulin preparations for intravenous application. I. Protein composition and antibody content. Vox Sang. 42 (1982) 62–73.
Römer, J., Späth, P. J., Skvaril, F., Nydegger, U. E. Characterization of various immunoglobulin preparations for intravenous application. II. Complement activation and binding to staphylococcus protein A. Vox Sang. 42 (1982) 74–80.
Kistler, P., Nitschmann, H. Large scale production of human plasma fractions. Vox Sang. 7 (1962) 414–424.
Stephan, W. Beseitigung der Komplementfixierung von γ-Globulin durch chemische Modifizierung mit β-Propiolacton. Z. Klin. Chem. Klin. Biochem. 7 (1969) 282–286.
Schultze, H. E., Schwick, G. Über neue Möglichkeiten intravenöser Gammaglobulin-Applikation. Dtsch. Med. Wochenschr. 87 (1962) 1643–1650.
Nisonoff, A. Enzymatic digestion of rabbit gamma globulin and antibody and chromatography of digestion products. Meth. Med. Res. 10 (1964) 134–141.
Mayer, M. M. Complement and complement fixation. In:Kabat, E. A., Mayer, M. M. (eds.): Experimental Immunochemistry, 2nd ed., Thomas Books, Springfield, IL 1964, pp. 133–240.
Herzberg, K., Reuss, K., Dahn, R. Vergleichende Immunitätsprüfung an Mäusen mit verschiedenen Influenza-Impfstoffen. Z. Hygiene 149 (1964) 481–496.
Bradley, J. V. Wilcoxon's Rank-Sum Test for identical populations, sensitive to unequal locations. In:Bradley J. V. (ed.): Distribution-free statistical tests. Prentice-Hall, Inc., Englewood Cliffs, NJ 1968, pp. 105–114.
Ronneberger, H., Vollerthun. R., Zwisler, O. Intrazelluläre Verteilung von intravenös verabreichbarem homologem Immunglobulin in der Leber. Die gelben Hefte 18 (1978) 156–158.
Vollerthun, R., Sedlacek, H. H., Ronneberger, H. Gewebeverteilung von nativem und enzymbehandeltem Human-Immunglobulin. Experimentelle Untersuchungen. Dtsch. Med. Wochenschr. 102 (1977) 684–686.
Kryzhanovsky, G. N. The mechanism of action of tetanus toxin: Effect of synaptic processes and some particular features of toxin binding by the nervous tissue. Naunyn-Schmiedeberg's Arch. Pharmacol. 276 (1973) 247–270.
Eibl, M., Friers, G. Hemmung der Opsonisierung vonE. coli durch enzymatisch gespaltene Gammaglobuline. Wien. Klin. Wochenschr. 90 (1980) 717–721.
Tympner, K.-D., Klose, P. K., Janka, G., Liegel, D. Einfluß intravenös verträglicher Immunglobuline auf die Phagozytoseleistung der Granulozytenin vitro. Münch. Med. Wschr. 120 (1978) 251–252.
Barandun, S., Castel, F., Makula, M. F., Morell, A., Plan, R., Skvaril, F. Clinical tolerance and catabolism of plasmin-treated gamma globulin for intravenous application. Vox Sang. 28 (1975) 157–175.
Ring, J., Duswald, K. H., Bachmann, T., Stephan, W., Brendel, W. Konzentration intravenös applizierter Gammaglobulin-Präparate in der Hundehaut. Arch. Dermatol. Res. 260 (1977) 201–206.
Glöckner, W. M., Sieberth, H. G., Eggers, H., Kurz, H. Hepatitisprophylaxe durch intravenöses Hyperimmunglobulin. Mitt. Arbeitsgem. Klin. Nephrol. 9 (1980) 100–105.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stephan, W., Dichtelmüller, H. Efficacy of intravenous immunoglobulin preparations against viral and bacterial infections in mouse protection tests. Infection 11, 227–231 (1983). https://doi.org/10.1007/BF01641203
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01641203