Summary
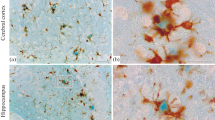
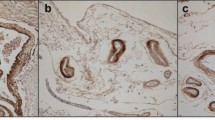
A one step en bloc silver staining method which was originally established to study nucleolar organizer regions has been applied for the demonstration of both paired helical filaments (PHF) and extracellular cerebral amyloids in semi-thin sections and at the electron microscopic level. The three forms of PHF can be visualized: (1) neurofibrillary tangles are shown in all stages from first appearance in form of intracellular patches of PHF to severely degenerated shadow-like “ghost” tangles; (2) neuropil threads are distinctly stained in great numbers; and (3) PHF are easily detected as neuritic components in amyloid plaques. All forms of fibrillar extracellular amyloid structures, i.e. “diffuse”, “classical” and “burnt out” plaques, are well demonstrated; congophilic angiopathy reveals amyloid preferentially in arteries and arterioles of the leptomeninges and cortex ranging from small circumscribed patches to large circumferential amounts with occasional plaque-like condensations or broad loose accumulations of amyloid; perivascular cuffs and laminar subpial deposits of amyloid are stained as well. At the electron microscopic level all lesions are clearly visible in non uranyl/lead-stained specimens, characterized by varying numbers of silver grains on a pale background. The detailed demonstration of structures in archival material, which had been stored in paraffin and re-embedded for electron microscopy, is due to the demonstration of argyrophilic structures by the protective colloidal developer of gelatin and formic acid and to the proteolytic resistance of insoluble PHF and extracellular amyloids in plaques and congophilic angiopathy.
Similar content being viewed by others
References
Beyreuther K, Masters CL (1991) Amyloid precursor protein (APP) andβA4 amyloid in the etiology of Alzheimer's disease: precursor product relationships in the derangement of neuronal function. Brain Pathol 1:241–251
Braak H, Braak E, Grundke-Iqbal I, Iqbal K (1986) Occurence of neuropil threads in the senile human brain and in Alzheimer's disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci Lett 65:351–355
Brumback RA, Feeback DL, Leech RA, Davis JJ (1991) Neuritic plaques and neurofibrillary tangles of Alzheimer's disease are resistant to proteolytic degradation following tissue infarction. Brain Pathol 1:317–318
Campbell SK, Switzer RC, Martin TL (1987) Alzheimer's plaques and tangles: a controlled and enhanced silver staining method. Soc Neurosci Abstr 13:678
Cross RB (1982) Demonstration of neurofibrillary tangles in paraffin sections: a quick and simple method using a modification of Palmgren's method. Med Lab Sci 39:67–69
Davies L, Wolska B, Hilbich C, Multhaup G, Martins R, Simms G, Beyreuther K, Masters CL (1988) A4 amyloid protein deposition and the diagnosis of Alzheimer's disease: prevalence in aged brains determined by immunohistochemistry compared with conventional neuropathologic techniques. Neurology 38:1688–1693
Defossez A, Beauvillain JC, Delacourte A, Mazucca M (1988) Alzheimer's disease: a new evidence for common epitopes between microtubule associated protein Tau and paired helical filaments (PHF): demonstration at the electron microscope level by double immunogold labeling. Virchows Arch [A] 413:141–145
Gallyas F (1971) Silver staining of Alzheimer's neurofibrillary changes by means of a physical development. Acta Morphol Hung 19:1–8
Gallyas F, Wolff JR (1986) Metal-catalyzed oxidation renders silver intensification selective. Application for the histochemistry of diaminobenzidine and neurofibrillary changes. J Histochem Cytochem 34:1667–1672
Glenner GG, Wong WC (1984) Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 122:1131–1135
Kidd M (1963) Paired helical filaments in electron microscopy of Alzheimer's disease. Nature 197:192–193
Kidd M (1964) Alzheimer's disease. An electron microscopic study. Brain 87:307–320
Kitamoto T, Ogomori K, Tateishi J, Prusiner SB (1987) Methods in laboratory investigations. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest 57:230–236
Masters CL, Multhaup G, Simms G, Pottgiesser J, Martins RN, Beyreuther K (1985) Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimers disease contain the same protein as the amyloid of plaque cores and vessels. EMBO J 4:2757–2763
Miyakawa T, Watanabe K, Katsuragi S (1986) Ultrastructure of amyloid fibrils in Alzheimer's disease and Down's syndrome. Virchows Arch [B] 52:99–106
Miyakawa T, Katsuragi S, Araki K, Hashimura T, Kimura T, Kuramoto R (1989) Ultrastructure of neurofibrillary tangles in Alzheimer's disease. Virchows Arch [B] 57:267–273
Ogomori K, Kitamoto T, Tateishi J, Sato Y, Suetsugu M, Abe M (1989) Beta-protein amyloid is widely distributed in the central nervous system of patients with Alzheimer's disease. Am J Pathol 134:243–251
Ploton D, Bobichon H, Adnet JJ (1982) Ultrastructural localization of NOR in nucleoli of human breast cancer tissues using a one-step Ag-NOR method. Biol Cell 43:229–232
Probst A, Langui D, Ulrich J (1991) Alzheimer's disease: a description of the structural lesions. Brain Pathol 1:229–239
Reusche E (1991) Silver staining of senile plaques and neurofibrillary tangles in paraffin sections- a simple and effective method. Pathol Res Pract 187:1045–1049
Schlote W (1965) Die Amyloidnatur der kongophilen, drusigen Entartung der Hirnarterien (Scholz) im Senium. Acta Neuropathol (Berl) 4:449–468
Shin RW, Ogomori K, Kitamoto T, Tateishi J (1989) Increased tau-accumulation in senile plaques as a hallmark in Alzheimer's disease. Am J Pathol 134:1365–1371
Terry RD, Gonatas NK, Weiss M (1964) Ultrastructural studies in Alzheimer's presenile dementia. Am J Pathol 44:269–283
Wisniewski H, Narang HK, Terry RP (1976) Neurofibrillary tangles of paired helical filaments. J Neurol Sci 27:173–182
Yamaguchi H, Nakazato Y, Shoji M, Ihara Y, Hirai S (1990a) Ultrastructure of the neuropil threads in the Alzheimer brain: their dendritic origin and accumulation in the senile plaques. Acta Neuropathol (Berl) 80:368–374
Yamaguchi H, Haga C, Nakazoto Y, Kosaka K (1990b) Distinctive, rapid and easy labeling of diffuse plaques in the Alzheimer brains by a new methenamine silver stain. Acta Neuropathol (Berl) 79:569–572
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reusche, E., Ogomori, K., Diebold, J. et al. Electron microscopic study of paired helical filaments and cerebral amyloid using a novel en bloc silver staining method. Vichows Archiv A Pathol Anat 420, 519–525 (1992). https://doi.org/10.1007/BF01600257
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01600257