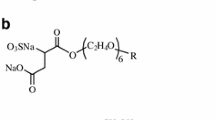
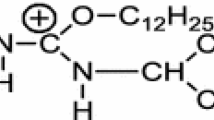
Summary
It has been found that adsorption isotherms describing the adsorption of sodium 4-hexadecyl-oxytolyl2-sulfonate and sodium dodecyl-benzene-4-sulfonate at different solid surfaces revealed maxima at the range of CMC. Similar maxima also occurred on the curves χ/c.
Adsorption of the mentioned surface-active substances from micellar solutions at a solid did not lead to establishment of an equilibrium which would result in an adsorption film containing micelles.
The decrease in adsorption at a solid in the range of CMC and above it is discussed on the basis of adsorption equilibrium, established between the micelle, the monomer, and the adsorption film. Postmicellar association of the surfactant can result in a minimum on the adsorption isotherm.
During adsorption of a surfactant from micellar solutions, solubilization can take place of reaction products of the surfactant and polyvalent inorganic cations or weakly dissociated molecules, in the rangec eq≧ CMC.
Zusammenfassung
Die Adsorptionsisothermen von Na-HexadecylOxytolyl-2-Sulfonat und Na-Dodecyl-Benzol-4-Sulfonat an verschiedenen festen Oberflächen haben im Bereich der CMC ein Maximum. Dieses Maximum wurde auch bei den Abhängigkeiten χ/c gefunden. Die Adsorption der untersuchten grenzflächenaktiven Stoffe aus ihren mizellaren Lösungen führt nicht zur Bildung eines Adsorptionsfilmes mit eingelagerten Mizellen.
Der Abfall der Adsorption der Tensidmoleküle im Bereich der CMC und darüber hinaus wurde aufgrund der Einstellung des Adsorptionsgleichgewichtes zwischen der Mizelle, dem Monomer und dem Adsorptionsfilm diskutiert. Die postmizellare Assoziation der Tensidmoleküle kann die Entstehung eines Minimums auf der Adsorptionsisotherme zur Folge haben.
Während der Adsorption des Tensids aus seinen mizellaren Lösungen können in Bereichenc eq ≥ CMC wenig dissoziierte Moleküle oder ein Reaktionsprodukt des Tensids mit den mehrwertigen anorganischen Kationen solubilisiert werden.
Similar content being viewed by others
References
Blazy, P., J. Cases, J. F. Delon, R. Houot, J. J. Prédali, andD. Vestier, Sciences de la Terre, Univ. Nancy, XIII, pp. 19–53 (1968).
Malik, W. U., S. K. Srivastava, andDevendra Gupta, Clay Minerals9, 369 (1973).
Schubert, H., W. Schneider, andH. Baldauf, Tenside6, 185 (1969).
Li, H. C. andP. L. Bruyn, Surface Sci.5, 203 (1966).
Brunauer, S., L. M. Deming, W. S. Deming, andE. Teller, J. Am. Chem. Soc.62, 1723 (1940).
Giles, H. C., T. H. MacEvan, S. N. Nakhwa, andD. Smith, J. Chem. Soc. 1960, 3973.
Vold, M. J. andA. K. Phansalkar, Rec. trav. chim.74, 41 (1955).
Corrin, M. L., E. L. Lind, A. Roginsky, andW. D. Harkins, J. Colloid Sci.4, 485 (1949).
Fava, A. andH. Eyring, J. Phys. Chem.62, 984 (1958).
Tamamushi, B. andK. Tamaki, Proc. Sec. Int. Congr. Surface Activity, Vol. III, p. 449 (London 1957).
Marian, J. E., Holzforschg.20, 91 (1966).
Gaudin, A. M. andD. W. Fuerstenau, Trans. Am. Inst. Min. Engs.202, 958 (1955).
Cook, M. A., J. Colloid Sci.28, 547 (1968).
Kitchener, J. A., J. Photogr. Sci.13, 152 (1965).
Dobiáš, B., J. Spurný, andE. Freudlová, Coll. Czechoslov. Chem. Commun.24, 3663 (1959).
Dobiáš, B., Freiberger ForschungshefteA 335, 7 (1964).
Dobiáš, B., Tenside-Detergents9, 322 (1972).
Epton, S. R., Trans. Faraday Soc.44, 226 (1948).
Bareš, M., J. Preiningerová, J. Cesenek, A. Spal, R. Fiala, andO. Holendová, Chem. průmysl21, 172 (1971).
Haul, R. andG. Dümbgen, Chemie-Ing-Technik,35, 586 (1963).
Dobiá, B, andE. Ujec, Tenside4, 252 (1967).
Davies, J. T. andE. K. Rideal, Interfacial Phenomena, p. 42 and 196 (New York, 1963).
Zimmels, Y. andI. J. Lin, Colloid & Polymer Sci.252, 594 (1974).
Trapnell, B. W. M., Chemisorption, p. 142. Izdatelstvo Inostrannoj Literatury (Moskva, 1958).
Dobiáš, B., Tenside Detergents13, 131 (1976).
Dobiáš, B., J. Spurny, andJ. Cibulka, Coll. Czechoslov. Chem. Commun.31, 166 (1966).
Ginn, M. E., Cationic Surfactants, p. 341 (New York, 1970).
Dobiáš B., in the press.
Elwortby, P. H., A. T. Florence, andC. B. Macfarlane, Solubilization by Surface-active Agents, p. 48 and 61 (London, 1968).
Zimmels, Y., I. J. Lin, andJ. P. Friend, Colloid & Polymer Sci.,253, 404 (1975).
Author information
Authors and Affiliations
Additional information
With 13 figures and 1 table
Rights and permissions
About this article
Cite this article
Dobiáš, B. Adsorption and electrokinetic phenomena in the system solid — micellar solution of a surface-active substance. Colloid & Polymer Sci 255, 682–690 (1977). https://doi.org/10.1007/BF01550057
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01550057