Summary
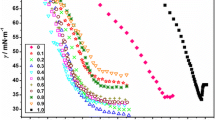
Stepwise bulk association is correlated to the stepwise pattern of some liquid-gas and solid-liquid interfacial parameters. On the basis of continuous distribution and aGibbs approach, a modifiedGibbs adsorption isotherm, applying to the liquid gas interface, is introduced. The latter describes the stepwise pattern of the liquid-gas interfacial tension curve. The behaviour of adsorption density, zeta potential, contact angle, settling rate of calcite suspensions and carbonate content in solutions were discussed in terms of a proposed stepwise adsorption model.
This model includes stages of direct adsorption of monomers as well as of submicelles (or micelles). Specific combinations of chemi- and physical adsorption may possibly explain distribution between different adsorbing species, thus showing in better detail the initial equilibrium between the different associated forms coexisting in solution. TheStern-Graham adsorption model is applied and a modified version introduced.
Zusammenfassung
Es besteht eine Korrektion zwischen der schrittweisen Assoziation in Masse und der schrittweisen Assoziation an Flüssigkeit-Gas- und Feststoff-Gas-Grenzflächen. Eine abgeänderteGibbssche Adsorptionsisotherme, auf der ununterbrochenenBoltzmannschen Verteilung und einerGibbsschen Betrachtungsweise beruhend und auf die Flüssigkeit-Gas-Grenzfläche anwendbar, wird vorgeschlagen. Diese Isotherme ist eine Darstellung des schrittweisen Schemas der Flüssigkeit-Gas-Grenzflächenspannungskurve. Die Verhaltensweisen der Adsorptionsdichte, des Zeta-Potentials, des Kontaktwinkels, der Absetzgeschwindigkeiten von Kalzitsuspensionen und des Karbonatgehaltes in Lösungen wurden unter dem Gesichtswinkel eines vorgeschlagenen schrittweisen Adsorptionsmodells erörtert.
Dieses Modell umfaßt Stadien von direkter Monomeradsorption sowie von Untermicellen (oder Micellen). Bestimmte Verbindungen von chemischer und physikalischer Adsorption könnten gegebenenfalls die Aufteilung unter verschiedene adsorbierende Arten erklären, so daß das anfängliche Gleichgewicht zwischen den in der Lösung gleichzeitig bestehenden verschiedenen assoziierten Formen mit genaueren Einzelheiten aufgezeigt werden kann. DasStern-Grahamsche Adsorptionsmodell wird angewandt und eine abgeänderte Fassung desselben eingeführt.
Similar content being viewed by others

References
Tamamushi, B. andK. Tamaki, Proceedings of the Second International Congr. Surface Activity. III, p. 449 (London 1957).
Corrin, Lind, Roginsky, andHarkins, J. Colloid Sci.4, 485 (1949).
Shinoda, K., Colloidal surfactants, Chap. 1 (New York-London 1963).
Mukerjee, P. andK. J. Mysels, Critical micelle concentration of aqueous surfactant systems (National Standard References Data System) NSRDS-NBS-36, USA (1971).
Mukerjee, P., Adv. Colloid Interface Sci.1, 241 (1967).
Ekwall, P., I. Danielsson, andP. Stenius, in: M.T.P. international review of science; surface chemistry and colloids (physical chemistry) series one, Vol. 7, 97 (Baltimore-London 1972).
Zimmels, Y. andI. J. Lin, Colloid & Polymer Sci.252, 594 (1974).
Gaudin, A. M. andD. W. Fuerstenau, Trans. AIME202, 958 (1955).
Somasundaran, P. andD. W. Fuerstenau, J. Phys. Chem.70, 90 (1966).
Wakamatsu, T. andD. W. Fuerstenau, Advan. Chem. Ser.79, 161 (1968).
Fuerstenau, D. W., T. W. Healy andP. Somasundaran, Trans. AIME229, 321 (1964).
Fuerstenau, D. W., Pure and Applied Chem.24, 1, 135 (1970).
Dick, S. G., D. W. Fuerstenau, andT. W. Healy, J. Colloid Interface Sci.37, 595 (1971).
Somasundaran, P. andD. W. Fuerstenau, Trans. AIME252, 275 (1972).
Cook, M. A., J. Phys. Chem.55, 383 (1951).
Rosen, M. J. andH. A. Goldsmith, Systematic analysis of surface-active agents (New York 1966).
Lin, I. J.andA. Metzer, J. Phys. Chem.75, 19, 3000 (1971).
Adam, N. K. andG. Jessop, J. Chem. Soc., 1863 (1925).
Lin, I. J., Trans., I.M.M.76, 288 (1967).
Somasundaran, P., J. Colloid Interface Sci.31, 4, 557 (1969).
Powney, J. andD. O. Jordan, Trans. Faraday Soc.34, 363 (1938).
Kling, W. andH. Lange, Proceedings of the Second International Congr. Surface Activity, I, 295 (1957).
Elworthy, P. H. andK. J. Mysels, J. Colloid Sci.21, 31 (1966).
Eigeles, M. A., Theoretical Basis of the Flotation of non Sulfide Minérals, Metallurgizdat (1950).
Cases, J. M., Surface Science9, 57 (1968).
Cases, J. M., VIII Congrès International de Préparation des Mineralés. Leningrad, S-13, 1 (1968).
Blazy, P., J. Cases, J. F. Delon, R. Houot, J. J. Predali, andD. Vestier, Science de la Terre. Nancy, Vol. 13, 1, 21 (1968).
Predali, J. J. andJ. M. Cases, Proc. 10th Int. Min. Proc. Congr., London (1973).
Good, R. J., Wetting S.C.I. Monograph No. 25 Society of Chemical Industry, p. 444 (1967).
Plitt, L. R. andM. K. Kim, Paper Presented at the 1972 CIM Conference Halifax, Aug. 1972.
Peck, A. S., U.S. Bur. Mines Report of Investigation No. 6202 (1963).
Fuerstenau, M. C. andD. J. Miller, Trans. AIME238, 153 (1967).
Berlinskii, A. I. andN. D. Klyneva, Chem. Abs.76, 74921S (1972).
Du Rietz, C., Proceedings of Second Scandinavian Symposium on Surface Activity, Stockholm Edited byP. Ekwall, K. Groth, andV. Runnström-Reio, p. 21 (1964).
Paterson, J. G., Trans. I.M.M.79, 91 (1970).
Read, A. D. andR. M. Manser, Warren Spring Laboratory LR 143 (MP), 1970.
Zisman, W. A., Advances in chemical series, 43, Contact angle, wettability and adhesion. p. 1 (Washington 1964).
Author information
Authors and Affiliations
Additional information
With 19 figures
Rights and permissions
About this article
Cite this article
Zimmels, Y., Lin, I.J. & Friend, J.P. The relation between stepwise bulk association and interfacial phenomena for some aqueous surfactant solution. Colloid & Polymer Sci 253, 404–421 (1975). https://doi.org/10.1007/BF01382160
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01382160