Summary
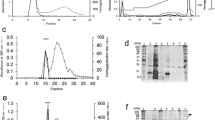
Expression of various sugar residues on the plasma membrane of frog (Rana perezi) epidermal cells at different stages of differentiation has been monitored with the use of a battery of HRP-conjugated lectins. In paraffin-embedded tissue, mannose residues (stained by Concanavalin A) were detected at the keratinocyte cell surface in all epidermal strata. However,Lens culinaris agglutinin (LCA), also specific for mannose, specifically stained the plasma membrane of cells from the stratum germinativum. Expression of N-acetyl-glucosamine (GlcNAc), labelled with wheat germ agglutinin (WGA), was maximum at the cell surface of basal cells and progressively decreased through the stratum spinosum. Galactose (Gal) and N-acetyl-galactosamine (GalNAc) residues, labelled withGriffonia simplicifolia I (GS I) andGlycine max (SBA) agglutinins, respectively, were expressed according to the degree of differentiation in amphibian epidermal cells. Sialic acid-containing glycoproteins, labelled withLimax flavus agglutinin (LFA), were found in the outermost plasma membrane of the replacement cell layer and stratum corneum. Glycoproteins responsible for the observed lectin-binding patterns have been identified by staining on nitrocellulose filters after electrophoresis of solubilized plasma membrane fractions and Western blotting. Changes at the level of glycosylation of plasma membrane glycoproteins as epidermal cells differentiate are discussed on the basis of a progressive addition of Gal residues. Integral membrane proteins have been solubilized with the non-denaturing detergent CHAPS and glycoproteins containing terminal Gal residues, that are expressed according to the degree of differentiation in frog epidermis, have been partially purified by affinity chromatography on a GS I-Sepharose 4 B column. The purified fraction was composed by four acidic glycoproteins with isoelectric points between 4.6 and 5.2 and, in SDS-gels gave five major protein bands with approximate molecular weights of 148, 140, 102, 60, and 52 kDa in SDS-gels. The 102 and 52 kDa bands correspond to the a and β subunits of amphibian epidermal Na+,K+-ATPase as demonstrated by specific staining with a polyclonal antibody against the catalytic subunit of pig kidney proton pump and staining with lectins GS I, GS II, and WGA. Possible relationships between higher molecular weight proteins and the constituents of intramembranous particles from the outermost plasma membranes of the replacement cell layer and the stratum corneum are also discussed.
Similar content being viewed by others
Abbreviations
- BSA:
-
bovine serum albumin
- CHAPS:
-
(3-[(cholamidopropyl) dimethyl-ammonio] 1-propanesulfonate)
- Con A:
-
Canavalia ensiformis agglutinin
- DTT:
-
dithiothreitol
- Gal:
-
galactose
- GalNAc:
-
N-acetyl-D-galactosamine
- GlcNAc:
-
N-acetyl-D-glucosamine
- GS I:
-
Griffonia simplicifolia agglutinin I
- GS II:
-
Griffonia simplicifolia agglutinin II
- HRP:
-
horseradish peroxidase
- LFA:
-
Limax flavus agglutinin
- LCA:
-
Lens culinaris agglutinin
- NDPAGIF:
-
non-denaturing polyacrylamide gel isoelectric focusing
- PAGE:
-
polyacrylamide gel electrophoresis
- PAP:
-
peroxidase-antiperoxidase
- PBS:
-
phosphate buffered saline
- PMSF:
-
phenyl methyl sulphonyl fluoride
- RCL:
-
replacement cell layer
- SBA:
-
soybean agglutinin (Glycine max)
- SB:
-
stratum basal
- SDS:
-
sodium dodecyl sulphate
- SG:
-
stratum granulosum
- SS:
-
stratum spinosum
- UEA I:
-
Ulex europaeus agglutinin I
- WGA:
-
wheat germ (Triticum vulgaris) agglutinin
References
Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD (1983) Molecular biology of the cell. Garland, New York
Berger EG, Buddecke E, Kamerling JP, Kobata A, Paulson JC, Vliegenthart JFG (1982) Structure, biosynthesis and functions of glycoprotein glycans. Experientia 38: 1129–1258
Brown D, Ilic V (1979) Freeze-fracture differences in plasma membrane of the stratum corneum and replacement layer cells of amphibian epidermis. J Ultrastruct Res 67: 55–64
—, Grosso A, De Sousa RC (1979) Intramembrane particle aggregates and water flow in amphibian epidermis. J Cell Biol 83: 248 a
— — — (1980) Isoproterenol-induced intramembrane particle aggregation and water flux in toad epidermis. Biochim Biophys Acta 596: 158–164
Bueno C, Navas P, Aijón J, López-Campos JL (1981) Glycoconjugates in the epidermis ofPleurodeles waltlii. J Ultrastruct Res 77: 354–359
Burón MI, García-Herdugo G, Moya L, Navas P (1987 a) Sodium pump as defined by K+-stimulatedp-nitrophenyl phosphate shows a gradient from inner to outer surface in frog epidermis. Epithelia 1: 75–83
— —, Navas P (1987 b) Lectin inhibition and kinetics of microsomal K+-dependentp-nitrophenyl phosphatase of frog epidermis. Comp Biochem Physiol 86B: 241–244
—, Navas P, García-Herdugo G, Morré DJ (1987 c) Isolation of plasma membrane from amphibian epidermis: evidence for a basal-to-apical charge and activity gradient. Eur J Cell Biol 44: 176–186
—, Rodríguez-Aguilera JC, González-Reyes JA, Villalba JM, Alcaín FJ, Navarro F, Navas P (1993) A quantitative ultrastructural and cytochemical study of TPA-induced differentiation in HL-60 cells. Leukemia Res 17: 863–872
Chevalier J, Adragna N, Bourguet J, Gobin R (1981) Fine structure of intramembranous particle aggregates in ADH-treated frog urinary bladder and skin: influence of glutaraldehyde and N-ethyl-maleimide. Cell Tissue Res 218: 595–606
De Sousa RC, Grosso A (1981) On water transport and amphibian epithelia. In: Bloch K, Bolis L, Tosteson DC (eds) Membranes, molecules, toxins and cells. John Wright, Bristol, pp 255–269
Dulley JR, Grieve PA (1975) A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem 64: 136–141
Elias PM (1981) Lipids and the epidermal permeability barrier. Arch Dermatol Res 270: 95–117
Fisher JA, Baxter-Lowe LA, Hokin LE (1984) Site of synthesis of the a and β subunits of the Na,K-ATPase in brine shrimp nauplii. J Biol Chem 259: 14217–14221
Frens G (1973) Controlled nucleation for the regulation of particle size in monodisperse gold suspensions. Nature 241: 20
Gallagher JT (1984) Carbohydrate-binding properties of lectins: a possible approach to lectin nomenclature and classification. Biosci Rep 4: 621–632
Garfin DE (1990) Isoelectric focusing. Methods Enzymol 182: 459–477
Glynn JM (1985) The Na+,K+-transporting adenosine triphosphatase. In: Martonosi AN (ed) The enzymes of biological membranes, vol 3. Plenum, New York, pp 35–114
Goldstein IJ, Hayes CE (1978) The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem 35: 127–340
Gurd JW, Mahler HR (1974) Fractionation of synaptic plasma membrane glycoproteins by lectin affinity chromatography. Biochemistry 13: 5193–5198
Kornfeld R, Kornfeld K (1980) Structure of glycoproteins and their oligosaccharide units. In: Lennarz WJ (ed) The biochemistry of glycoproteins and proteoglycans. Plenum, New York, pp 11–34
Kornfeld K, Reiman RL, Kornfeld R (1981) The carbohydratebinding specificity of pea and lentil lectins-fucose is an important determinant. J Biol Chem 256: 6633–6640
Lavker RM (1974) Horny cell formation in the epidermis ofRana pipiens. J Morphol 142: 365–378
Merril CR, Goldman D, Van Keuren ML (1984) Gel protein stains: silver stain. Methods Enzymol 104: 441–447
Mogilnaya G, Shubich M (1982) Histochemical basis of the vertebrate epidermis differentiation in the comparative aspects. Acta Histochem 70: 214–223
Navas P, Villalba JM, García-Herdugo G (1985) Carbohydrate cytochemistry on the cell surface coat of amphibian keratinocytes during differentiation. Z Mikrosk Anat Forsch 99: 447–454
— —, Burón MI, García-Herdugo G (1987) Lectins as markers for plasma membrane differentiation of amphibian keratinocytes. Biol Cell 60: 225–234
Nemanic MK, Whitehead JS, Elias PM (1983) Alterations in membrane sugars during epidermal differentiation. J Histochem Cytochem 31: 887–897
Ookusa Y, Takata K, Nagashima M, Hirano H (1983) Distribution of glycoconjugates in normal human skin using biotinyl lectins and avidin-horseradish peroxidase. Histochemistry 79: 1–7
Rittman BR, McKenzie IC, Rittman GA (1982) Lectin binding to murine oral mucose and skin. Arch Oral Biol 27: 1013–1019
Roth J (1983) Application of lectin-gold complexes for electron microscopic localization of glycoconjugates on thin sections. J Histochem Cytochem 31: 987
Sato M, Yonezawa S, Uehara H, Arita Y, Sato E, Muramatsu T (1986) Differential distribution of receptors for two fucose-binding lectins in embryos and adult tissues of the mouse. Differentiation 30: 211–219
Stöckbauer P, Gahmberg CG, Andersson LC (1985) Changes in cell surface glycoproteins and antigens during differentiation of the human myeloid leukemia cell lines ML-1, ML-2 and HL-60. Cancer Res 45: 2821–2826
Villalba JM, Navas P (1989) Polarization of plasma membrane glycoconjugates in amphibian epidermis during metamorphosis. Histochemistry 90: 453–458
— —, García-Herdugo G (1987) Lectin binding patterns in amphibian epidermis. Acta Histochem 81: 51–57
—, Burón MI, Roldán JM, Navas P (1993 a) Plasma membrane glycoproteins during anuran amphibian epidermal development. Protoplasma 172: 136–144
—, Roldán JM, Navas P (1993 b) Flask cells and flask-shaped glandular cells of amphibian skin specifically produce fucose-rich glycoproteins. Histochemistry 99: 363–367
Wieser RJ, Oesch F (1988) Contact-dependent regulation of growth of diploid human fibroblasts is dependent upon the presence of terminal galactose residues on plasma membrane glycoproteins. Exp Cell Res 176: 80–86
Weibel ER (1979) Practical methods for biological morphometry. In: Weibel ER (ed) Stereological methods, vol 1. Academic Press, London, pp 101–159
Zaccone G, Fasulo S, Lo Cascio P, Licata A, Ainis L (1986) Patterns of enzyme activities related to epithelial differentiation in the amphibian epidermis. Arch Biol (Brussels) 97: 223–236
Zieske JD, Bernstein LA (1982) Modification of cell surface glycoprotein: addition of fucosyl residues during epidermal differentiation. J Cell Biol 95: 626–631
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Villalba, J.M., Navarro, F., Roldán, J.M. et al. Expression of carbohydrate residues in plasma membrane glycoproteins during the differentiation of amphibian epidermal cells. Protoplasma 178, 87–96 (1994). https://doi.org/10.1007/BF01545959
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01545959