Abstract
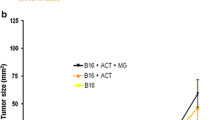
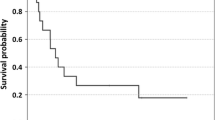
High-dose interleukin-(IL-2) has been broadly studied in tumour therapy, yet it may be inhibitory to T-cell-dependent immunity. Therefore immune and tumour responses mediated by low-dose IL-2 were studied systematically with respect to the feedback organisation of immune responses. IL-2 was administered once daily at three dose levels: 0.18, 0.9, 4.5 MIU/m2 according to three different schedules requiring subcutaneous (s.c.) injection once weekly (four doses, stratum I), thrice weekly every other day (nine doses, stratum II), or five times weekly every other week (ten doses, stratum III). A total of 46 patients with advanced cancer were randomly assigned to one of the nine treatment groups. Systemic effects were induced at doses as low as 0.18 MIU/m2 IL-2 s.c. as demonstrated from measurable IL-2 serum levels, induction of circulating IL-6, a transient lymphopenia, and stimulation of delayed-type hypersensitivity (DTH) responses of the skin. Analysis of the different IL-2 schedules demonstrated (a) prolonged effects of once-weekly injections on DTH responses, lymphocyte and eosinophil counts, and (b) maximum increase of eosinophil counts and preferential expansion of activated NK cells with repeated injections every 48 h or 72 h (stratum II), while sequential treatment according to stratum III was found to be more potent in increasing the number of activated T cells. A tumour response was observed in 1/15 patients with renal cell carcinoma who experienced more than 50% tumour regression for 8 months; 12 patients had stable disease for 4 months (median). These data demonstrate prolonged immunological effects of ultra-low doses of s. c. IL-2 despite its short half-life. Furthermore, scheduling of IL-2 was found to affect immune responsiveness specifically as demonstrated by the differential effects on natural killer and T cell populations.
Similar content being viewed by others
References
Bocci V (1991) Interleukins. Clinical pharmacokinetics and practical implications. Clin Pharmacokinet 21:274–284
Brink MRM van den, Boggs SS, Herbermann RB, Hiserodt JC (1990) The generation of natural killer (NK) cells from NK precursor cells in rat long-term bone marrow cultures. J Exp Med 172: 303–313
Cantrell DA, Smith KA (1983) The transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med 158: 1895–1905
Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA (1982) Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med 155:1823–1841
Klein J (1990) Regulation of the immune response. In: Klein J (ed.) Immunology. Blackwell: Boston, pp 386–391
Kolitz JE, Mertelsmann R (1991) The immunotherapy of human cancer with interleukin-2: present status and future directions. Cancer Invest 9:529–542
Konrad MW, Hemstreet G, Hersh EM, Mansell PWA, Mertelsmann R, Kolitz JE, Bradley EC (1990) Pharmacokinetic of recombinant interleukin 2 in humans. Cancer Res 50:2009–2017
Lenardo MJ (1991) Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature 353:858–861
Lindemann A, Herrmann F, Oster W, Mertelsmann R (1989) Lymphokine activated killer cells. Blut 59:375–384
Lotze MT, Matory YL, Ettinghausen SE, Rayner AA, Sharrow SO, Seipp CAY, Custer MC, Rosenberg SA (1985) In vivo administration of purified human interleukin 2. J Immunol 135:2865–2875
McElrath MJ, Kaplan G, Burkhardt RA, Cohn ZA (1990) Cutaneous response to recombinant interleukin 2 in human immunodeficiency virus 1-seropositive individuals. Proc Natl Acad Sci USA 87: 5783–5787
Mulé JJ, Shu S, Rosenberg SA (1985) The anti-tumor efficacy of lymphokine-activated killer cells and recombinant interleukin 2 in vivo. J Immunol 135:646–652
Nagler A, Lanier LL, Cwirla S, Phillips JH (1989) Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol 143:3183–3191
Pahwa R, Chatila T, Pahwa S, Paradise C, Day NK, Geha R, Schwartz SA, Slade H, Oyaizu N, Good RA (1989) Recombinant interleukin 2 therapy in severe combined immuno-deficiency disease. J Immunol 86:5069–5073
Philips JH, Gemlo BT, Myers WW, Rayner AA, Lanier LL (1987) In vivo and in vitro activation of natural killer cells in advanced cancer patients undergoing combined recombinant interleukin-2 and LAK cell therapy. J Clin Oncol 5:1933–1941
Rand TH, Silberstein DS, Kornfeld H, Weller PF (1991) Human eosinophils express functional interleukin 2 receptors. J Clin Invest 88:825–832
Soiffer RJ, Murray Ch, Cochran K, Cameron Ch, Wang E, Schow PW, Daley JF, Ritz J (1992) Clinical and immunologic effects of prolonged infusion of low-dose recombinant interleukin-2 after autologous and T-cell-depleted allogeneic bone marrow transplantation. Blood 79:517–526
Wiebke EA, Rosenberg SA, Lotze MT (1988) Acute immunologic effects of interleukin-2 therapy in cancer patients: decreased delayed type hypersensitivity response and decreased proliferative response to soluble antigens. J Clin Oncol 6:1440–1449
Yamaguchi Y, Suda T, Shiozaki H, Miura Y, Hitoshi Y, Tominaga A, Takatsu K, Kasahara T (1990) Role of IL-5 in IL-2-induced eosinophilia: in vivo and in vitro expression of IL-5 mRNA by IL-2. J Immunol 145:873–877
Yamaguchi Y, Suda T, Ohta S, Tominaga K, Miura Y, Kasahara T (1991) Analysis of the survival of mature human eosinophils: interleukin-5 prevents apoptosis in mature human eosinophils. Blood 78: 2542–2547
Author information
Authors and Affiliations
Additional information
Supported by Bundesministerium für Forschung und Technologie, Förderkennzeichen 01GA8901/3 to R. M.
Rights and permissions
About this article
Cite this article
Lindemann, A., Brossart, P., Höffken, K. et al. Immunomodulatory effects of ultra-low-dose interleukin-2 in cancer patients: a phase-IB study. Cancer Immunol Immunother 37, 307–315 (1993). https://doi.org/10.1007/BF01518453
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01518453