Abstract
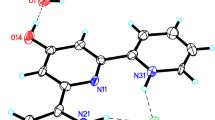
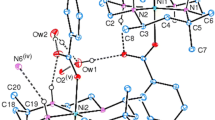
The diamagnetictrans-diene thiocyanate salt of the macrocyclic hexamethyl-1,4,8,11-tetraazacyclotetradecadienenickel(II) complex crystallizes in the triclinic system with unit cell dimensions:a = 7·334(4),b = 8·808(6),c = 21·04(2) Å; α = 63·7(0·9), β = 103·8(1·4), γ = 110·2(1·3) °; space groupP¯1;Z = 2. The positions of all 62 atoms of the formula unit C16H32N4Ni(SCN)2.H2O have been determined and refined by least-squares methods toR= 0·071 for 3536 X-ray diffractometric reflections. The optical activity of the two amine groups yields a racemic isomer. The only local symmetry element of the macrocyclic complex is the twofold rotation axis with the N-H bonds oriented on the same side of the approximate ligand plane. Both the C(CH3)2 and the imine groups are in atrans-configuration in the ring. The space group ensures that the crystal is a racemate, containing equal numbers of both enantiomers. One of the (SCN)− groups is at a distance Ni-S 3·28 Å, the other one is 4·65 Å. No rotational disorder of the CH3 groups has been observed. The three C-H bonds of the CH3 groups are in approximately antiprismatic orientations with respect to the three σ-bonds in the case of bonding to a C(sp3) atom. The average of the C(sp3)-C(sp2) bond lengths is 1·53 Å, and the mean value for the C(sp3)-C(sp2) bonds is 1·50 Å, with 3σ = 0·03 to 0·04 Å. The distances N(sp2)-C(sp3) 1·47 Å and 1·48 Å are significantly longer than the bond lengths N(sp2)=C(sp2) of 1·28 and 1·30 Å.
Similar content being viewed by others
References
Bailey, M. F. & Maxwell, I. E. (1966)Chem. Comm. 908.
Curtis, N. F., Curtis, Y. M. & Powell, H. K. J. (1966)J. Chem. Soc. (A), 1015.
Hazell, R., Nyborg, J., Danielsen, J. & Lauesen, S. (1966) Set of crystallographic programs. Unpublished results.
Hughes, E. W. (1941)J. Amer. Chem. Soc. 63, 1737.
Jehn, W. (1967)Z. anorg. allg. Chem. 351, 260.
Kilbourn, B. T., Ryan, R. R. & Dunitz, J. D. (1969)J. Chem. Soc. (A), 2408.
Warner, L. G., Rose, N. J. & Busch, D. H. (1967)J. Amer. Chem. Soc. 89, 703.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hanic, F., Mikloš, D. Stereochemistry of racemic hexamethyl-1,4,8,11-tetraazacyclotetradeca-4,11-dienenickel(II) thiocyanate, C16H32N4Ni(SCN)2 · H2O. Journal of Crystal and Molecular Structure 2, 115–124 (1972). https://doi.org/10.1007/BF01464792
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01464792