Conclusions
-
1.
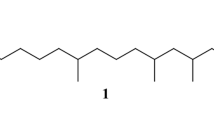
The effective synthesis of optically pure (4R,8R)-(−)-4,8-dimethyldecanal, the aggregational pheromone of meal worms, and of its Synergist, (4R,8S)-(+)-4,8-dimethyldecanal, is described.
-
2.
The readily available (R)-(+)-4-methyl-5-acetoxyvaleric acid was used for the first time as a chiral source for the synthesis of optically active natural compounds.
Similar content being viewed by others
Literature cited
T. Suzuki, Agric. Biol. Chem.,45, 2641 (1981).
K. Mori, S. Kuwahara, and H. Ueda, Tetrahedron,39, 2439 (1983).
K. Mori, M. Kato, and S. Kuwahara, Leibigs Ann. Chem., 861 (1985).
T. Suzuki, J. Kozaki, R. Sugawara, and K. Mori, Appl. Entomol. Zool.,19, 15 (1984).
R. Brettle and F. S. Holland, J. Chem. Soc., 4836 (1962).
R. A. Sheldon and J. K. Kochi, in: Organic Reactions, Vol. 19, Wiley, New York (1972), p. 279.
G. Piankatelli and A. Scettri, Gazz. Chim. Ital.,104, 343 (1974).
R. G. Almquist, J. Craze, G. Jennings-White, et al., J. Med. Chem.,25, 1292 (1982).
A. M. Moiseenkov, I. M. Zaks, and B. S. Él'yanov, Zh-Obshch. Khim.,53, 1260 (1983).
D. E. Dorman, M. Jautelat, and J. D. Roberts, J. Org. Chem.,36, 2757 (1971).
J. W. De Haan and I. J. M. Van de Ven, Org. Magn. Reson.,5, 147 (1973).
W. J. Houlihan, Perfum. Essent. Oil Record.,52, 781 (1962).
M. M. Martin and J. G. MacConnell, Tetrahedron,26, 307 (1970).
K. Mori, Tetrahedron,30, 3817 (1974).
M. Kobayashi, T. Minamizawa, and H. Mitsuhashi, Steroids,29, 823 (1977).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 4, pp. 865–871, April, 1988.
Rights and permissions
About this article
Cite this article
Cheskis, B.A., Lebedeva, K.V. & Moiseenkov, A.M. Effective synthesis of (4r,8r)- and (4r,8s)-enantiomers of 4,8-dimethyldecanal, the aggregational pheromone of the beetlesTeibolium confusum andTribolim castaneum . Russ Chem Bull 37, 745–751 (1988). https://doi.org/10.1007/BF01455492
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01455492