Summary
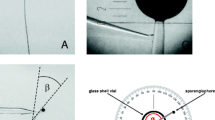
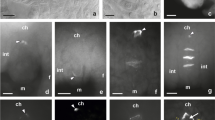
GerminatingCochliobolus sativus spores were induced to form appressoria on a variety of artificial surfaces, including replicas of the barley leaf surface. Evidence was obtained for the involvement of chemical and topographic signals during induction of appressorium formation inC. sativus. Germ tube thigmotropism was also observed in vitro. Ultrastructure relevant to appressorium formation was observed, including the germ tube apex, apical swelling of the germ tube apex prior to appressorium formation, the appressorium with associated septation and the penetration peg. Cytochemical probes applied to germlings at the electron microscope level failed to detect α-D-mannan, α-D-glucan, β-D-galactan, D-glcNAc or D-galNAc polymers in the extracellular mucilage associated with the fungal germlings. The ultrastructure of hyphal apices from germlings grown under different nutritional conditions differed with respect to Spitzenkörper morphology, apex shape and in the quantity of associated extracellular mucilage. Experimental findings are discussed relative to current understanding of appressorium induction in more extensively studied systems.
Similar content being viewed by others
Abbreviations
- PDA:
-
potato dextrose agar
- DS:
-
dilute salts
- Con:
-
A concanavalin A
- RcA120 :
-
Ricinus communis agglutinin120
- WGA:
-
wheat germ agglutinin
- HpA:
-
Helix pomatia agglutinin
- DIC:
-
differential interference contrast
- UV:
-
ultraviolet
- TEM:
-
transmission electron microscopy
- NNF:
-
National Nanofabrication Facility
References
Armentrout VM, Downer AJ, Grasmick DL, Weinhold AR (1987) Factors affecting infection cushion development byRhizoctonia solani on cotton. Phytopathology 77: 623–630
Benhamou N (1989) Preparation and application of lectin-gold complexes. In: Hyatt MA (ed) Colloidal gold: principles, methods, and applications, vol 1. Academic Press, San Diego, pp 95–143
Bourett TM, Howard RJ (1990) In vitro development of penetration structures in the rice blast fungusMagnaporthe grisea. Can J Bot 68: 329–342
Carlson H, Stenram U, Gustafson M, Jansson H-B (1991) Electron microscopy of barley root infection by the fungal pathogenBipolaris sorokiniana. Can J Bot 69: 2724–2731
Clark CA, Lorbeer JW (1976) Comparative histopathology ofBotrytis squamosa andB. cinerea on onion leaves. Phytopathology 66: 1279–1289
Clay RP, Benhamou N, Fuller MS (1991) Ultrastructural detection of polysaccharides in the cell walls of two members of the Hyphochytriales. Mycol Res 95: 1057–1064
Endo RM, Amacher RH (1964) Influence of guttation fluid on infection structures ofHelminthosporium sorokinianum. Phytopathology 54: 1327–1334
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold solutions. Nature 241: 20–22
Frossard R (1981) Effect of guttation fluids on growth of microorganisms on leaves. In: Blakeman JP (ed) Microbial ecology of the phylloplane. Academic Press, New York, pp 213–226
Fuller MS, Jaworski A (eds) (1987) Zoosporic fungi in teaching and research. Southeastern Publishing, Athens, GA
Hau FC, Rush MC (1979) Leaf surface interactions betweenCochliobolus miyabeanus and susceptible and resistant rice cultivars. Phytopathology 69: 527
Hoch HC, Howard RJ (1980) Ultrastructure of freeze-substituted hyphae of the basidiomyceteLaetisaria arvalis. Protoplasma 103: 281–297
—, Staples RC (1991) Signaling for infection structure formation in fungi. In: Cole GT, Hoch HC (eds) The fungal spore and disease initiation in plants and animals. Plenum, New York, pp 25–42
— —, Bourett T (1987 a) Chemically induced appressoria inUromyces appendiculatus are formed aerially, apart from the substrate. Mycologia 79: 418–424
— —, Whitehead B, Comeau J, Wolf ED (1987 b) Signaling for growth orientation and cell differentiation by surface topography inUromyces. Science 235: 1659–1662
Howard RJ, Ferrari MA (1989) Role of melanin in appressorium function. Exp Mycol 13: 403–418
Huang HC, Tinline RD (1976) Histology ofCochliobolus sativus infection in subcrown internodes of wheat and barley. Can J Bot 54: 1344–1354
Jannson H-B, Johansson T, Nordbring-Hertz B (1988) Chemotropic growth of germ tubes ofCochliobolus sativus to barley roots or root exudates. Trans Br Mycol Soc 90: 647–650
Johnson T (1934) A tropic response in germ tubes of urediospores ofPuccinia graminis tritici. Phytopathology 24: 80–82
Kwon YH, Hoch HC (1991) Temporal and spatial dynamics of appressorium formation inUromyces appendiculatus. Exp Mycol 15: 116–131
— —, Staples RC (1991) Cytoskeletal organization inUromyces urediospore germling apices during appressorium formation. Protoplasma 165: 37–50
Mims CW, Richardson EA (1989) Ultrastructure of appressorium development by basidiospore germlings of the rust fungusGymnosporangium juniperi-virginianae. Protoplasma 148: 111–119
Reymond OL, Pickett-Heaps J (1983) A routine flat embedding method for electron microscopy of microorganisms allowing selection and precisely oriented sectioning of single cells by light microscopy. J Microsc 130: 79–84
Reynolds ES (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol 17: 208–212
Roberson RW, Fuller MS (1988) Ultrastructural aspects of the hyphal tip ofSclerotium rolfsii preserved by freeze substitution. Protoplasma 146: 143–149
Rowley JC III, Moran DT (1975) A simple procedure for mounting wrinkle-free sections on formvar coated slot grids. Ultramicroscopy 1: 151–155
Staples RC, Macko V, Wynn WK, Hoch HC (1983) Terminology to describe differentiation response by germlings of fungal spores. Phytopathology 73: 380
—, Hassouna S, Hoch HC (1985) Effect of potassium on sugar uptake and assimilation by bean rust germlings. Mycologia 77: 248–252
Stumpf MA, Leinhos GME, Staples RC, Hoch HC (1991) The effect of pH and K+ on appressorium formation byUromyces appendiculatus uredospore germlings. Exp Mycol 15: 356–360
Wynn WK (1976) Appressorium formation over stomates by the bean rust fungus: response to a surface contact stimulus. Phytopathology 66: 136–146
—, Staples RC (1981) Tropisms of fungi in host recognition. In: Staples RC, Toenniessen GA (eds) Plant disease control: resistance and susceptibility. Wiley, New York, pp 45–69
Yadav BS (1981 a) Behavior ofCochliobolus sativus during its infection of barley and wheat leaves. Aust J Bot 29: 71–79
— (1981 b) Factors affecting spore germination ofCochliobolus sativus over host leaf surface. Isr J Bot 30: 81–87
—, Mandahar CL (1981) Secretion of cytokinin-like substances in vivo and in vitro byHelminthosporium sativum and their role in pathogenesis. Z Pflanzenk Pflanzenschutz 88: 726–733
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Clay, R.P., Enkerli, J. & Fuller, M.S. Induction and formation ofCochliobolus sativus appressoria. Protoplasma 178, 34–47 (1994). https://doi.org/10.1007/BF01404119
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01404119