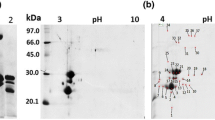
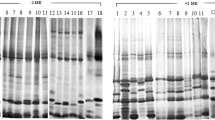
Summary
Major sclerotial polypeptides from the psychrophiles,Myriosclerotinia borealis (W 51),Coprinus psychromorbidus (LRS 131),Typhula idahoensis (W 21), andTyphula incarnata (W 21) were purified by using polyacrylamide gel electrophoresis and electroelution. Polyclonal antibodies were raised against these major sclerotial polypeptides. Immunofluorescence microscopy showed that the major sclerotial polypeptides from all four psychrophilic species were sequestered in discrete protein bodies of cultured and field-grown sclerotia. Western blot analysis indicated that all antisera reacted positively with their respective antigens, the major sclerotial polypeptides. Reciprocal immunological cross-reactions were observed between the major sclerotial polypeptides ofM. borealis (W 51) andT. idahoensis (W 21). Antiserum to the major sclerotial polypeptides of bothM. borealis andT. idahoensis also recognized the major sclerotial polypeptides ofC. psychromorbidus (LRS 131). It is suggested that the major sclerotial polypeptides of these psychrophilic plant pathogens may act as storage proteins.
Similar content being viewed by others
Abbreviations
- W 51:
-
Myriosclerotinia borealis (W 51)
- LRS 131:
-
Coprinus psychromorbidus (LRS 131)
- W 21:
-
Typhula idahoensis (W 21)
- W 29:
-
Typhula incarnata (W 29)
- anti W 51:
-
antiserum to the major sclerotial polypeptide ofM. borealis W 51
- anti LRS 131:
-
antiserum to the major sclerotial polypeptides ofC. psychromorbidus (LRS 131)
- anti W 21:
-
antiserum to the major sclerotial polypeptides ofT. idahoensis (W 21)
- anti W 29:
-
antiserum to the major sclerotial polypeptides ofT. incarnata (W 29)
- SDS:
-
sodium dodecylsulfate
- kDa:
-
kilodalton
- PAGE:
-
polyacrylamide gel electrophoresis
- HRP:
-
horseradish peroxidase
- PBS:
-
phosphate buffered saline
- TBS:
-
Tris buffered saline
- FITC:
-
fluorescein isothiocyanate
References
Baszczynski CL (1986) Immunochemical analysis of heat-shock protein synthesis in maize (Zea mays L.). Can J Genet Cytol 28: 1076–1087
Bendayan M (1984) Concentration of amylase along its secretory pathway in the pancreatic acinar cell as revealed by high resolution immunocytochemistry. Histochem J 16: 85–108
Bhown AS, Mole JE, Hunter F, Bennett JC (1980) High-sensitivity sequence determination of proteins quantitatively recovered from sodium dodecyl sulfate gels using an improved electrodialysis procedure. Anal Biochem 103: 184–190
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248
Bullock S, Ashford AE, Willetts HJ (1980 a) The structure and histochemistry of sclerotia ofSclerotinia minor Jagger. II. Histochemistry of extracellular substances and cytoplasmic reserves. Protoplasma 104: 333–351
—,Willetts HS, Ashford AE (1980 b) The structure and histochemistry of sclerotia ofSclerotinia minor Jagger. I. Light and electron microscope studies on sclerotial development. Protoplasma 104: 315–331
—,Willetts HJ, Ashford EA (1983) The structure and histochemistry of sclerotia ofSclerotinia minor Jagger. III. Changes in ultrastructure and loss of reserve materials during carpogenic germination. Protoplasma 117: 214–225
Comings DE, Tack LC (1972) Similarities in the cytoplasmic proteins of different organs and species examined by SDS gel electrophoresis. Exp Cell Res 75: 73–78
Fisher DB (1968) Protein staining of ribboned epon sections for light microscopy. Histochemistry 16: 92–96
Grenville DJ, Peterson RL, Piche Y (1985 a) The development, structure, and histochemistry of sclerotia of ectomycorrhizal fungi. I. Pisolithus tinctorius. Can J Bot 63: 1402–1411
— — — (1985 b) The development, structure, and histochemistry of sclerotia of ectomycorrhizal fungi. II. Paxillus involutus. Can J Bot 63: 1412–1417
Insell JP, Huner NPA, Newsted WJ, Van Huystee RB (1985) Light microscopic and polypeptide analyses of sclerotia from mesophilic andf psychrophilic pathogenic fungi. Can J Bot 63: 2305–2310
Johnson GD, De C. Nogueira-Araujo GM (1981) A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods 43: 349–350
Kelley PM, Schlesinger MJ (1982) Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol 2: 267–274
Kalousek F, Darigo MD, Rosenberg LE (1980) Isolation and characterization of propionyl-CoA carboxylase from normal human liver: evidence for a protomeric tetramer of nonidentical subunits. J Biol Chem 255: 60–65
Laemmli VK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Lott JNA (1980) Protein bodies. In:Tolbert NE (ed) The biochemistry of plants. A comprehensive treatise, voll, the plant cell. Academic Press, New York, pp 589–623
Newsted WJ (1987) Major sclerotial polypeptides of psychrophilic fungi: identification, immunological relatedness, localization andin vivo synthesis. Ph.D. Thesis, The University of Western Ontario
—,Huner NPA (1987) Major polypeptides associated with differentiation in psychrophilic fungi. Can J Bot 65: 233–241
- - (1988) Major sclerotial polypeptides of psychrophilic fungi: temperature regulation ofin vivo synthesis in vegetative hyphae. Can J Bot, in press
— —,Insell JP, Griffith M, Van Huystee RB (1985) The effects of temperature on the growth and polypeptide composition of several snow mold species. Can J Bot 63: 2311–2318
Petersen GR, Russo M, Van Etten JL (1982) Identification of major proteins in sclerotia ofSclerotinia minor andSclerotinia trifoliorum. Exp Mycol 6: 268–273
Reed JE, Chollet R (1985) Immunofluorescent localization of phosphoenolpyruvate carboxylase and ribulose 1,5-biphosphate carboxylase/oxygenase proteins in leaves of C3, C4 and C3-C4 intermediateFlaveria species. Planta 165: 439–445
Russo GM, Dahlberg KR, Van Etten JL (1982) Identification of a development-specific protein in sclerotia ofSclerotinia sclerotiorum. Exp Mycol 6: 259–267
—,Van Etten JL (1985) Synthesis and localization of a development-specific protein inSclerotinia sclerotiorum. J Bacteriol 163: 696–703
Spitzer E, Lott JNA (1982) Protein bodies in umbelliferous seeds. I. Structure. Can J Bot 60: 1381–1391
Tsang VC, Peralta JM, Simons AR (1983) Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol 92: 377–391
Willetts HJ, Bullock S (1982) Studies on the ontogeny and ultrastructure of the sclerotium ofBotrytis cinerea Pers. ex Nocca & Balbis. Can J Microbiol 28: 1347–1354
Zilinskas BA, Howell DA (1987) Immunological conservation of phycobilisome rod linker polypeptides. Plant Physiol 85: 322–326
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Newsted, W.J., Huner, N.P.A. Major sclerotial polypeptides of psychrophilic pathogenic fungi: Intracellular localization and antigenic relatedness. Protoplasma 147, 162–169 (1988). https://doi.org/10.1007/BF01403344
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01403344