Summary
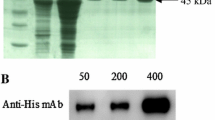
The gene encoding protein p10, a structural protein of African swine fever (ASF) virus, has been mapped, sequenced and expressed inE. coli. Protein p10 was purified from dissociated virus by reverse-phase HPLC, and its NH2-terminal end identified by automated Edman degradation. To map the gene encoding protein p10, a mixture of 20-mer oligonucleotides based upon a part of the amino acid sequence was hybridized to cloned ASF virus restriction fragments. This allowed the localization of the gene in fragmentEco RI K of the ASF virus genome. The nucleotide sequence obtained from this region revealed an open reading frame encoding 78 amino acids, with a high content of Ser and Lys residues. Several of the Ser residues are found in Ser-rich regions, which are also found in some nucleic acid-binding proteins. The gene coding for protein p10 has been inserted in an expression vector which contains the promoter for T 7 RNA polymerase. The recombinant plasmid was used to produce the ASF virus protein inE. coli. The bacterially produced p10 protein shows a strong DNA binding activity with similar affinity for both double-stranded and single-stranded DNA.
Similar content being viewed by others
References
Aguado B, Viñuela E, Alcamí A (1991) African swine fever virus fatty acid acylated proteins. Virology 185: 942–945
Almendral JM, Blasco R, Ley V, Beloso A, Talavera A, Viñuela E (1984) Restriction site map of African swine fever virus DNA. Virology 133: 258–270
Ballard DW, Philbrick WM, Bothwell ALM (1988) Identification of a novel 9-kDa polypeptide from nuclear extracts. J Biol Chem 263: 8450–8457
Baum JA, Geever R, Giles NH (1987) Expression of qa-1 F activator protein: identification of upstream binding sites in the qa gene cluster and localization of the DNA-binding domain. Mol Cell Biol 7: 1256–1266
Blasco R, López-Otín C, Muñoz M, Bockamp E, Simón-Mateo C, Viñuela E (1990) Sequence and evolutionary relationships of African swine fever virus thymidine kinase. Virology 178: 301–304
Carrascosa AL, del Val M, Santarén JF, Viñuela E (1985) Purification and properties of African swine fever virus. J Virol 54: 337–344
Chase JW, Williams KR (1986) Single-stranded DNA binding proteins required for DNA replication. Annu Rev Biochem 55: 103–136
Enjuanes L, Carrascosa AL, Moreno MA, Viñuela E (1976) Titration of African swine fever virus. J Gen Virol 32: 471–477
Esteves A, Graca R, Costa JV (1987) DNA-binding proteins specified by African swine fever virus. Virology 161: 403–409
Falaschi A, Cobianchi F, Riva S (1980) DNA-binding proteins and DNA-unwinding enzymes in eukaryotes. Trends Biochem Sci 5: 154–157
Garner MM, Revzin A (1981) A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of theEscherichia coli lactose operon regulatory system. Nucleic Acids Res 9: 3047–3060
Geider K, Hoffman-Berling H (1981) Proteins controlling the helical structure of DNA. Annu Rev Biochem 50: 233–260
González A, Talavera A, Almendral JM, Viñuela E (1986) Hairpin loop structure of African swine fever virus DNA. Nucleic Acids Res 14: 6835–6844
Grunstein M, Hogness DS (1975) Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci USA 72: 3961–3965
Hewick RM, Hunkapiller MW, Hood LE, Dreyer WJ (1981) A gas-liquid-solid phase peptide and protein sequenator. J Biol Chem 256: 7990–7997
Hope IA, Struhl K (1985) GCN4 protein, synthesized in vitro, binds HIS3 regulatory sequences: implications for general control of amino acid biosynthetic genes in yeast. Cell 43: 177–188
Hope IA, Struhl K (1986) Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell 46: 885–894
Hunkapiller MW, Lujan E, Ostander F, Hood LE (1983) Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Method Enzymol 91: 227–236
Kafatos FC, Jones CW, Efstratiadis A (1979) Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res 7: 1541–1552
Kumar A, Williams KR, Szer W (1986) Purification and domain structure of core hnRNP proteins A1 and A2 and their relationship to single-stranded DNA binding proteins. J Biol Chem 261: 11266–11273
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T 4. Nature 227: 680–685
Ley V, Almendral JM, Carbonero P, Beloso A, Viñuela E, Talavera A (1984) Molecular cloning of African swine fever virus DNA. Virology 133: 249–257
López-Otín C, Simón-Mateo C, Martínez L, Viñuela E (1989) Gly-Gly-X, a novel consensus sequence for the proteolytic processing of viral and cellular proteins. J Biol Chem 264: 9107–9110
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, New York
Mateucci MD, Caruthers MH (1981) Synthesis of deoxyoligonucleotides on a polymer support. J Am Chem Soc 103: 3185–3191
Messing J, Vieira J (1982) A new pair of M 13 vectors for selecting either strand of double digest restriction fragments. Gene 19: 269–276
Nakabeppu Y, Nathans D (1989) The basic region of Fos mediates specific DNA binding. EMBO J 8: 3833–3841
Rosenberg AH, Lade BN, Chui D, Lin S, Dunn JJ, Studier FW (1987) Vectors for selective expression of cloned DNAs by T 7 RNA polymerase. Gene 56: 125–135
Salas ML, Salas J, Viñuela E (1988) Phosphorylation of African swine fever virus proteins in vitro and in vivo. Biochimie 70: 627–635
Sanger F, Nicklen S, Coulsen AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467
Soma G, Kitahara N, Andoh T (1984) Molecular cloning and characterization of a cDNA clone for a protein specifically expressed in embryo as well as in chemically induced pancreatic B cell tumor of rat. Biochem Biophys Res Comm 124: 164–171
Studier WF, Moffat BA (1986) Use of bacteriophage T 7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol 189: 113–130
Tabarés E, Martínez J, Martin E, Escribano JM (1983) Proteins specified by African swine fever virus. Arch Virol 77: 167–180
Tabor S, Richardson CC (1985) A bacteriophage T 7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA 82: 1074–1078
Viñuela E (1987) Molecular biology of African swine fever virus. In: Becker Y (ed) African swine fever. Martinus Nijhoff, Boston, pp31–49
Walker JM, Gooderham K, Hastings JRB, Mayes E, Johns EW (1980) The primary structure of non histone chromosomal proteins HMG1 and 2. FEBS Lett 122: 264–270
Walker JM, Goodwin GH, Johns EW (1979) The primary structure of nucleosome associated chromosomal protein HMG14. FEBS Lett 100: 394–398
Williams KR, Lo Presti MB, Setoguchi M, Konigsberg W (1980) Amino acid sequence of the T4 DNA helix-destabilizing protein. Proc Natl Acad Sci USA 77: 4614–4617
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Muñoz, M., Freije, J.M.P., Salas, M.L. et al. Structure and expression inE. coli of the gene coding for protein p10 of African swine fever virus. Archives of Virology 130, 93–107 (1993). https://doi.org/10.1007/BF01318999
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01318999