Abstract
Beak and feather disease virus (BFDV) belongs to the family Circoviridae. A rolling-circle replication strategy based on a replication-associated protein (Rep) has been proposed for BFDV. The Rep gene of BFDV was expressed and purified, and it was shown to cleave short oligonucleotides containing the conserved nonanucleotide sequence found in the replication origin of circoviruses. This endonuclease activity was most efficient in the presence of the divalent metal ions Mg2+ and Mn2+. Rep proteins containing mutation in the ATPase/GTPase motifs and the 14FTLNN18, 61KKRLS65, 89YCSK92, and 170GKS172 motifs lacked endonuclease activity. The endonuclease activity was not affected by ATPase inhibitors, with the exception of N-ethylmaleimide (NEM), or by GTPase inhibitors, but it was decreased by treatment with the endonuclease inhibitor L-742001. Both the ATPase and GTPase activities were decreased by site-directed mutagenesis and deletion of the ATPase/GTPase and endonuclease motifs. The Rep protein was able to bind a double-stranded DNA fragment of P36 (dsP36) containing the stem-loop structure of the replication origin of BFDV. All of the Rep mutant proteins showed reduced ability to bind this fragment, suggesting that all the ATPase/GTPase and endonuclease motifs are involved in the binding. Other than NEM, all ATPase, GTPase, and endonuclease inhibitors inhibited the binding of the Rep protein to the dsP36 fragment. This is the first report describing the endonuclease activity of the Rep protein of BFDV.










Similar content being viewed by others
References
Rahaus M, Wolff MH (2003) Psittacine beak and feather disease: a first survey of the distribution of beak and feather disease virus inside the population of captive psittacine birds in Germany. J Vet Med B Infect Dis Vet Public Health 50:368–371
Bassami MR, Berryman D, Wilcox GE, Raidal SR (1998) Psittacine beak and feather disease virus nucleotide sequence analysis and its relationship to porcine circovirus, plant circovirus, and chicken anemia virus. Virology 249:453–459
Tood D (2000) Circovirus: immunosuppressive threats to avian species: a review. Avian Pathol 29:373–394
Varsani A, Regnard GL, Bragg R, Hitzeroth II, Rybicki EP (2011) Global genetic diversity and geographical and host-species distribution of beak and featherdisease virus isolates. J Gen Virol 92:752–767
Huang SW, Chiang YC, Chin CY, Tang PC, Liu PC, Wang CY (2016) The phylogenetic and recombinational analysis of beak and feather disease virus Taiwan isolates. Arch Virol 161:2969–2988
Hsu CM, Ko CY, Tsai HJ (2006) Detection and sequence analysis of avian polyomavirus and psittacine beak and feather disease virus from psittacine birds in Taiwan. Avian Dis 50:348–353
Steinfeldt T, Finsterbusch T, Mankertz A (2007) Functional analysis of cis- and trans-acting replication factors of porcine circovirus type 1. J Virol 81:5696–5704
Clerot D, Bernardi F (2006) DNA helicase activity is associated with the replication initiator protein Rep of tomato yellow leaf curl geminivirus. J Virol 80:11322–11330
Huang SW, Liu HP, Chen JK, Shien YW, Wong ML, Wang CY (2016) Dual ATPase and GTPase activity of the replication-associated protein (Rep) of beak and feather disease virus. Virus Res 213:149–161
Orozco BM, Miller AB, Settlage SB, Hanley-Bowdoin L (1997) Functional domains of a geminivirus replication protein. J Biol Chem 272:9840–9846
Vega-Rocha S, Byeon IL, Gronenborn B, Gronenborn AM, Campos-Olivas R (2007) Solution structure, divalent metal and DNA binding of the endonuclease domain from the replication initiation protein from porcine circovirus 2. J Mol Biol 367:473–487
Laufs J, Schumacher S, Geisler N, Jupin I, Gronenborn B (1995) Identification of the nicking tyrosine of geminivirus Rep protein. FEBS Lett 377:258–262
Steinfeldt T, Finsterbusch T, Mankertz A (2001) Rep and Rep’ protein of porcine circovirus type 1 bind to the origin of replication in vitro. Virology 291:152–160
Campos-Olivas R, Louis JM, Clérot D, Gronenborn B, Gronenborn AM (2002) The structure of a replication initiator unites diverse aspects of nucleic acid metabolism. Proc Natl Acad Sci USA 99:10310–10315
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
Benkert P, Biasini M, Schwede T (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27:343–350
Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Klefer F, Gallo Cassarino T, Bertoni M, Bordoli L, Schwede T (2014) SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42:W252–W258
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Remmert M, Biegert A, Hauser A, Soding J (2011) HHblits: lightning-fast iterative protein sequence searching by HMM–HMM alignment. Nat Methods 9:173–175
Hertig S, Goddard TD, Johnson GT, Ferrin TE (2015) Multidomain assembler (MDA) generates models of large multidomain proteins. Biophys J 108:2097–2102
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Abbate EA, Berger JM, Botchan MR (2004) The X-ray structure of the papillomavirus helicase in complex with its molecular matchmaker E2. Genes Dev 18:1981–1996
Cherney BW, Chaudhry B, Bhatia K, Butt TR, Smulson M (1991) Expression and mutagenesis of human poly(ADP-ribose) polymerase as a ubiquitin fusion protein from Escherichia coli. Biochemistry 30:10420–10427
Erdmann S, Scheele U, Garrett RA (2011) AAA ATPase p529 of Acidianus two-tailed virus ATV and host receptor recognition. Virology 421:61–66
Boer R, Russi S, Guasch A, Lucas M, Blanco AG, Perez-Luque R, Coll M, de la Cruz F (2006) Unveiling the molecular mechanism of a conjugative relaxase: the structure of TrwC complexed with a 27-mer DNA comprising the recognition hairpin and the cleavage site. J Mol Biol 358:857–869
Tewary SK, Liang L, Lin Z, Lynn A, Cotmore SF, Tattersall P, Zhao H, Tang L (2015) Structures of minute virus of mice replication initiator protein N-terminal domain: insights into DNA nicking and origin binding. Virology 476:61–71
Trempe F, Gravel A, Dubuc I, Wallaschek N, Collin V, Wallaschek N, Collin V, Gilbert-Girard S, Morissette G, Kaufer BB, Flamand L (2015) Characterization of human herpesvirus 6A/B U94 as ATPase, helicase, exonuclease and DNA-binding proteins. Nucleic Acids Res 43:6084–6098
Londono A, Riego-Ruiz L, Arguello-Astorga GR (2010) DNA-binding specificity determinants of replication proteins encoded by eukaryotic ssDNA viruses are adjacent to widely separated RCR conserved motifs. Arch Virol 155:1033–1046
Su D, Delaplane S, Luo M, Rempel DL, Vu B, Kelley MR, Gross ML, Georgiadis MM (2011) Interaction of APE1 with a redox inhibitor: Evidence for an alternate conformation of the enzyme. Biochemistry 50:82–92
Zhan X, Tan CK, Scott WA, Mian AM, Downey KM, So AG (1994) Catalytic distinct conformation of the ribonuclease H of HIV-1 reverse transcriptase by substrate cleavage patterns and inhibition by azidothymidylate and N-ethylmaleimide. Biochemistry 33:1366–1372
Mikhailov VS, Vanarsdall AL, Rohrmann GF (2008) Isolation and characterization of the DNA-binding protein (DBP) of the Autographa californica multiple nucleopolyhedrovirus. Virology 370:415–429
DuBios RM, Slavish PJ, Baughman BM, Yun MK, Bao J, Webby RJ, Webb TR, White SW (2012) Structural and biochemical basis for development of influenza virus inhibitors targeting the PA endonuclease. PLoS Pathog 8:e1002830
Yan Z, Zhang L, Fu H, Wang Z, Lin J (2014) Design of the influenza virus inhibitors targeting the PA endonuclease using 3D-QSAR modeling, side-chain hopping, and docking. Bioorg Med Chem Lett 24:539–547
Acknowledgements
This work was supported by Grants from the Ministry of Science and Technology, Taiwan (MOST 105-2313-B-005-030 and MOST 106-2313-B-005-053-MY3), and the iEGG and Animal Biotechnology Center from the Feature Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Tim Skern.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
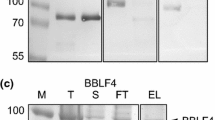
Expression and purification of the SUMO-Rep and Rep proteins using the recombinant pETite N-His SUMO Kan plasmid. M, molecular weight marker. Proteins from bacterial lysates of the expressed SUMO-Rep without (−) (lane 1) and with (+) IPTG-induction (lane 2) and the purified SUMO-Rep (lane 3) were resolved using SDS-PAGE (A). The Rep protein, after the removal of the SUMO-tag, was analyzed using SDS-PAGE (B). The Rep protein was probed with anti-Rep monoclonal antibody by Western blotting (C) (PDF 97 kb)
Supplementary Fig. 2
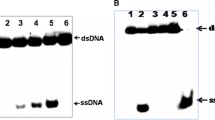
(A) The untagged Rep protein cleaves and covalently binds the P10 and P12 oligonucleotides through its endonuclease activity. The red arrowheads indicate the Rep protein-oligonucleotides adducts. M, molecular weight markers. (B) The replication origin of BFDV with a stem-loop structure followed by two repeated iteron sequences (5′-GGGGCACC/T-3′) is shown. The dsDNA as probes in the EMSA assay are indicated by a grey shading for dsP36 and by double arrows for dsP16 and dsP26, and the nucleotide numbers are indicated. (C) binding of the Rep protein without the tag to the dsP36 fragment, as measured by EMSA. The arrowheads and asterisks indicate the free probes and the Rep-dsP36 complexes, respectively (PDF 52 kb)
Supplementary Fig. 3
The endonuclease activities of the Rep Δ51-55 (Rep-box-2-del) and Rep Y55A (Rep-box-2-mut) proteins for both P10 and P12 oligonucleotides are lower than that of the Rep protein. The red arrowheads indicate the Rep protein-oligonucleotides adducts (A). The relative ATPase (B) and GTPase (C) activities of the Rep Δ51-55 (Rep-box-2-del) protein and Rep Y55A (Rep-box-2-mut) protein are higher than that of the Rep protein. The relative ATPase and GTPase activities of the Rep protein were set as 100%, and significant differences are indicated by a p-value of <0.05. The Rep Δ51-55 (Rep-box-2-del) protein and Rep Y55A (Rep-box-2-mut) protein showed stronger binding to the dsP36 DNA when compared to the Rep protein (D). The arrowheads and asterisks indicate the free probes and the Rep-dsP36 complexes, respectively (PDF 244 kb)
Rights and permissions
About this article
Cite this article
Chen, JK., Hsiao, C., Wu, JS. et al. Characterization of the endonuclease activity of the replication-associated protein of beak and feather disease virus. Arch Virol 164, 2091–2106 (2019). https://doi.org/10.1007/s00705-019-04292-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-019-04292-z