Summary
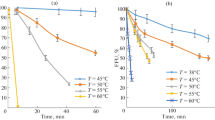
We have measured the infectivity of influenza A virus strains grown either in embryonated eggs or in chick embryo cells in culture after treatment at low pH. At pH values at which hemolysis occurs there was an irreversible loss of infectivity. The threshold pH, at which the infectivity was lost, depended on the hemagglutinin subtype of the virus strain. All H5 and H7 strains tested were extremely labile at low pH. In contrast, all H3 strains were relatively stable, independent of the species from which the viruses were isolated. With several H1 viruses the hemagglutination (HA) activity was irreversibly lost at intermediate pH values causing inactivation of infectivity. Strains with noncleaved hemagglutinins were much more stable. These observations might explain why duck influenza viruses can easily survive in lake water and wet faeces, and multiply in the intestinal tract, where trypsin is present. There are also significant differences in heat stability exhibited by influenza A strains. In contrast to pH stability this is not a specific trait of the hemagglutinin, since it can be influenced by reassortment. There is no correlation between the stability of infectivity at low pH and heat.
Similar content being viewed by others
References
Appleyard, G., Maber, H. B.: Plaque formation by influenza viruses in the presence of trypsin. J. gen. Virology25, 351–357 (1974).
Bosch, F. X., Orlich, M., Klenk, H.-D., Rott, R.: The structure of the hemagglutinin, a determinant for the pathogenicity of influenza viruses. Virology95, 197–207 (1979).
Hinshaw, V. S., Webster, R. G., Turner, B.: Waterborne transmission of influenza A viruses? Intervirology11, 66–68 (1979).
Hinshaw, V. S., Webster, R. G., Naeve, C. W., Murphy, B. R.: Altered tissue tropism of human-avian reassortant influenza viruses. Virology128, 260–263 (1983).
Hosaka, Y., Seriburi, O., Moran, M. G., Yasuda, Y., Fukai, K., Nerome, K.: Haemolysis and fusion by influenza viruses with heat inactivated neuraminidase activity. Biken J.25, 51–62 (1982).
Huang, R. T., Rott, R., Klenk, H.-D.: Influenza virus causes haemolysis and fusion of cells. Virology110, 243–247 (1981).
Klenk, H.-D., Rott, R., Becht, H.: On the structure of the influenza virus envelope. Virology47, 579–591 (1972).
Lenard, J., Baily, C. A., Miller, D. K.: pH dependence of influenza A virus-induced hemolysis is determined by the haemagglutinine gene. J. gen. Virol.62, 353–355 (1982).
McCahon, D., Schild, G. C.: An investigation of some factors affecting cross-reactivation between influenza A viruses. J. gen. Virol.12, 207–219 (1971).
Meda, T., Ohnishi, S.-I.: Activation of influenza virus by acidic media causes hemolysis and fusion of erythrocytes. FEBS-Lett.122, 283–287 (1980).
Maeda, T., Kawasaki, K., Ohnishi, S.-I.: Interaction of influenza virus hemagglutinin with target membrane lipids is a key step in virus induced hemolysis and fusion at pH=5.2. Proc. Natl. Acad. Sci. U.S.A.78, 4133–4137 (1981).
Matlin, K. S., Reggio, H., Helenius, A., Simons, K.: Infection entry pathway of influenza virus in a canine kidney cell line. J. Cell Biol.91, 601–603 (1981).
Rott, R., Reinacher, M., Orlich, M., Klenk, H.-D.: Cleavability of hemagglutinin determines spread of avian influenza viruses in the chorioallantoic membrane of chicken embryo. Arch. Virol.65, 123–133 (1980).
Sato, S. B., Kawasaki, K., Ohnishi, S.-I.: Hemolytic activity of influenza virus hemagglutinin glycoproteins activated in mildly acidic environments. Proc. Natl. Acad. Sci. U.S.A.80, 3153–3157 (1983).
Skehel, J. J., Bayley, P. M., Brown, E. B., Martin, S. R., Waterfield, M. D., White, J. M., Wilson, I. A., Wiley, D. C.: Changes in the conformation of the influenza virus hemagglutinin at the pH optimum of virus mediated fusion. Proc. Natl. Acad. Sci. U.S.A.79, 968–972 (1982).
Webster, R. G., Yakhno, M., Hinshaw, V. S., Bean, W. J., Murti, G.: Intestinal influenza: Replication and characterization of influenza viruses in ducks. Virology84, 268–276 (1978).
White, J., Helenius, A., Gething, M. J.: Haemagglutinin of influenza virus expressed from a cloned gene promotes membrane fusion. Nature300, 658–659 (1982).
Yoshimura, A., Kuroda, K., Kawasaki, K., Yamashima, S., Maeda, T., Ohnishi, S.: Infectious cell entry mechanism of influenza virus. J. Virol.43, 284–293 (1982).
Author information
Authors and Affiliations
Additional information
With 3 Figures
Rights and permissions
About this article
Cite this article
Scholtissek, C. Stability of infectious influenza A viruses to treatment at low pH and heating. Archives of Virology 85, 1–11 (1985). https://doi.org/10.1007/BF01317001
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01317001