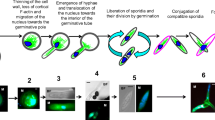
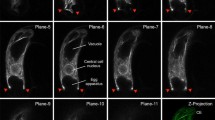
Summary
The role of F-actin in cell differentiation ofUromyces appendiculatus (bean rust fungus) germlings was examined by treating differentiating and nondifferentiating germlings with the actin-binding drugs cytochalasin E (CE) and phalloidin. Prolonged exposure of urediospores to 5×10−3–5 × 10−5 M CE induced nuclear division in up to 28–45% of the resulting germlings, whereas the rate of mitosis in established germlings exposed to these concentrations of CE was significantly lower (4–11%). Germlings treated with CE shifted from polarized apical growth to spherical expansion, cytoplasmic microfilaments were depolymerized, and nuclear inclusions became enlarged. Differentiating germlings exposed to a 10 minute pulse of 5×10−6M CE before the initiation of septum formation prevented the establishment of the F-actin septal ring and growth of the crosswall delimiting the appressorium. Although these CE treatments resulted in morphological and nuclear events similar to those occurring during normal appressorium formation, transient microfilament depolymerization was not sufficient to induce differentiation. Phalloidin stabilized cytoplasmic microfilaments, especially posteriorly-located microfilaments, but did not affect differentiation, nor did it significantly inhibit the effects of CE.
Similar content being viewed by others
Abbreviations
- CE:
-
cytochalasin E
- DAPI:
-
4,6-diamidino-2-phenylindole
- DMSO:
-
dimethyl sulfoxide
- F-actin:
-
filamentous actin
References
Bradley MO (1973) Microfilaments and cytoplasmic streaming: inhibition of streaming with cytochalasin. J Cell Sci 12: 327–343.
French RC, Graham CL, Gale AW, Long RK (1977) Structural and exposure time requirements for chemical stimulation of germination of uredospores ofUromyces phaseoli. J Agric Food Chem 25: 84–88
Geiger B (1985) Microfilament-membrane interaction. TIBS 10: 456–461
Grove SN, Sweigard JA (1980) Cytochalasin A inhibits spore germination and hyphal tip growth inGilbertella persicaria. Exp Mycol 4: 239–250
Heath IB, Heath MC (1979) Structural studies of the development of the development of infection structures of cowpea rust,Uromyces phaseoli varvignae. II. Vacuoles. Can J Bot 57: 1830–1837
Herth W, Franke WW, Van der Woude WJ (1972) Cytochalasin stops tip growth in plants. Naturwissenschaften 59: 38–39
Himes R., Kersey R, Ruscha M, Houston L (1976) Cytochalasin A inhibits thein vitro polymerization of brain tubulin and muscle actin. Biochem Biophys Res Commun 68: 1362–1370
Hoch HC, Bourett TM, Staples RC, 1986: Inhibition of cell differentiation inUromyces with D2O and taxol. Eur J Cell Biol 41: 290–297
—,Mitchell JE (1972) A continuous flow system for inducing and observing asexual spore formation inAphanomyces euteiches. Can J Bot 50: 681–682
—,Staples RC (1983 a) Ultrastructural organization of the nondifferentiated uredospore germling ofUromyces phaseoli varietytypica. Mycologia 75: 795–824
— — (1983 b) Visualization of actinin situ by rhodamineconjugated phalloin in the fungusUromyces phaseoli. Eur J Cell Biol 32: 52–58
— — (1984) Evidence that cAMP initiates nuclear division and infection structure formation in the bean rust fungus,Uromyces phaseoli. Exp Mycol 8: 37–46
— — (1985) The microtubule cytoskeleton in hyphae ofUromyces phaseoli germlings: Its relationship to the region of nucleation and to the F-actin cytoskeleton. Protoplasma 124: 112–122.
Kropf DL (1986) Electrophysiological properties ofAchlya hyphae: Ionic currents studied by intracellular potential recording. J Cell Biol 102: 1209–1216
Lin S, Spudich JA (1974) Biochemical studies on the mode of action of cytochalasin B. J Biol Chem 249: 5778–5783
Macko VR, Staples RC, Gershon H, Renwick JAA (1971) Self-inhibitor of bean rust uredospores: methyl,3,4-dimethoxy-cinnamate. Science 170: 539–540
Maclean-Fletcher S, Pollard TD (1980) Mechanism of action of cytochalasin B on actin. Cell 20: 329–341
Manavanthu EK, Thomas D, Des S (1980) Selectivity of cytochalasin A as a respiratory inhibitor. FEMS Microbiol Lett 7: 199–202
— — (1983) Cytochalasin A and respiratory inhibition in the water moldAchlya ambisexualis. Can J Bot 29: 15–20
Mascarenhas JP, Lafountain J (1972) Protoplasmic streaming, cytochalasin B and growth of the pollen tube. Tissue and Cell 4: 11–14
Nothnagel EA, Barak LS, Sanger JW, Webb WW (1981) Fluorescence studies on modes of cytochalasin B and phallotoxin action on cytoplasmic streaming inChara. J Cell Biol 88: 364–372
Piction JM, Steer MW (1981) Determination of secretory vesicle production rates by dictyosomes in pollen tubes ofTradescantia using cytochalasin D. J Cell Sci 49: 261–272
— — (1985) The effects of ruthenium red, lanthanum, fluorescein isothiocyanate and trifluoperazine on vesicle transport, vesicle fusion and tip extension in pollen tubes. Planta 163: 20–26
Reiss H-D, Grime GW, Li MQ, Takacs J, Watt F (1985) Distribution of elements in the lily pollen tube tip, determined with the Oxford scanning proton microprobe. Protoplasma 126: 147–152
—,Herth (1979) Calcium ionophore A 23187 affects localized wall secretion in the tip region of pollen tubes ofLilium longiflorum. Planta 145: 225–232
Schroeder TE (1970) The contractile ring, I. Fine structure of dividing mammalian (HeLa) cells and the effects of cytochalasin B. Z Zellforsch 109: 431–449
Staples RC (1974) Synthesis of DNA during differentiation of bean rust uredospores. Physiol Plant Pathol 4: 415–424
—,App AA, Ricci P (1975) DNA synthesis and nuclear division during formation of infection structures by bean rust uredospore germlings. Arch Microbiol 104: 123–127
—,Grambow H-J, Hoch HC (1983 a) Potassium ion induces rust fungi to develop infection structures. Exp Mycol 7: 40–46
— — —,Wynn WK (1983 b) Contact with membrane grooves induces wheat stem rust uredospore germlings to differentiate appressoria but not vesicles. Phytopathology 73: 1436–1439
—,Gross D, Tiburzy R, Hoch HC, Hassouna S, Webb WW (1984) Changes in DNA content of nuclei in rust uredospore germlings during the start of differentiation. Exp Mycol 8: 245–255
—,Hoch HC (1982) A possible role for microtubules and microfilaments in the induction of nuclear division in bean rust uredospore germlings. Exp Mycol 6: 293–302
- -(1984) A sensing mechanism in rust uredospore germlings responsive to host morphology starts the cell cycle. In:Roberts DW,Aist JR (eds) Infection processes of fungi. The Rockefeller Foundation, pp 126–136
Thomas D, Des S (1978) Cytochalasin effects in plants and eukaryotic microbial systems. In:Tannenbaum SW (ed) Cytochalasins: Biochemical and cell biological aspects. Frontiers in Biology vol 46. North-Holland Pub. Co., Amsterdam, pp 257–275
Wessells NK, Spooner BS, Ash JF, Bradley MO, Luduena MA, Taylor EL, Wrenn JT, Yamada KM (1971) Microfilaments in cellular and developmental processes. Science 171: 135–143
Wynn WK (1976) Appressorium formation over stomates by the bean rust fungus: response to surface contact stimulus. Phytopathology 66: 136–146
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tucker, B.E., Hoch, H.C. & Staples, R.C. The involvement of F-actin inUromyces cell differentiation: The effects of cytochalasin E and phalloidin. Protoplasma 135, 88–101 (1986). https://doi.org/10.1007/BF01277002
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01277002