Abstract
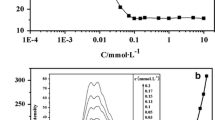
Cationic, anionic and non-ionic surfactants adsorb readily from aqueous solution on to Amberlite XAD 4. The ionic surfactants cause the pH of the solution in contact with the resin to differ from that in the bulk of solution, cationic surfactants increasing the interfacial pH and anionic surfactants decreasing it. This causes a shift in the pH transition interval of a co-adsorbed pH indicator when measured with respect to the bulk solution. The quantity of ionic surfactant adsorbed tends to a constant value (presumably monolayer coverage) with increasing solution concentration, this amount being a function of the individual surfactant, whereas non-ionic surfactants readily form multilayers. Significant adsorption occurs when the surfactant possesses at least 14 carbon atoms.
Similar content being viewed by others
References
O. S. Wolfbeis,Fresenius' Z. Anal. Chem. 1986,325, 387.
W. R. Seitz,CRC Crit. Rev. Anal. Chem. 1988,19, 135.
W. R. Seitz,Comput. Meth. Prog. Biomed. 1989,30, 9.
D. C. Ashworth, R. Narayanaswamy,Comput. Meth. Prog. Biomed. 1989,30, 21.
J. I. Petersen, S. R. Goldstein, R. V. Fitzgerald, D. K. Buckhold,Anal. Chem. 1980,52, 864.
G. F. Kirkbright, R. Narayanaswamy, N. A. Welti,Analyst 1984,109, 1025.
M. C. Moreno, A. Martinez, P. Millan, C. Camara,J. Mol. Struct. 1986,143, 553.
M. Bacci, F. Baldini, A. M. Scheggi,Anal. Chim. Acta 1988,207, 343.
D. W. Fuerstenau,J. Phys. Chem. 1956,60, 981.
Y. Gao, C. Vue, S. Lu, W. Gu, T. Gu,J. Coll. Interf. Sci. 1984,100, 58.
Y. Gao, J. Du, T. Gu,J. Chem. Soc. Faraday Trans, 1 1987,83, 2671.
B.-Y. Zhu, T. Gu,J. Chem. Soc. Faraday Trans. 1 1989,85, 3813.
S. M. Ahmed, M. S. El-Aasser, F. J. Micale, G. W. Paehlein, J. W. Vanderhoff,Froc. Sect. 52nd Colloid Surf. Sci. Symp. 1978,2, 853 (C.A.92, 23005u).
B. Kronberg, M. Lindstrom, P. Stenius,ACS Symp. Ser. 1986,311, 225 (C.A. 105, 117019r).
D. C. Ashworth, H. P. Huang, R. Narayanaswamy,Anal. Chim. Acta 1988,213, 251.
R. Narayanaswamy, F. Sevilla,Analyst 1986,111, 1085.
S. M. S. Al-Amir, D. C. Ashworth, R. Narayanaswamy, R. E. Moss,Talanta 1989,36, 645.
P. Kubelka, F. Munk,Z. Tech. Physik. 1931,12, 321.
B. W. Bailey, J. E. Chester, R. M. Dagnall, T. S. West,Talanta 1968,15, 1359.
G. S. Hartley,Trans. Faraday Soc. 1934,30, 444.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ashworth, D.C., Narayanaswamy, R. The effect of surfactants on the pH transition interval of bromothymol blue immobilized on XAD 4. Mikrochim Acta 106, 287–292 (1992). https://doi.org/10.1007/BF01242101
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01242101