Abstract
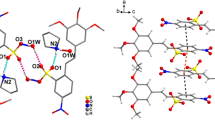
10,10′-(5H,5′H)-spirobiphenophosphazinium chloride is one of the few examples of a spirophosphonium salt reported in the literature. This compound crystallized in space groupP21/c witha = 12·293(7),b = 13·279(8),c = 17·56(1) Å, β = 130·43(3) ° andZ = 4. The finalR index was 0·057. The results of the X-ray analysis indicated that this phosphorus heterocycle may indeed possess aromatic character. This contention was supported in solution by a31 P nmr study.
Similar content being viewed by others
References
Azaroff, L. V. (1955)Acta Crystallogr. 8, 701.
Bak, B., Christensen, D., Hansen, L. & Rastrup-Andersen, J. (1956)J. Chem. Phys. 24, 720.
Bak, B., Hansen-Nygaard, L. & Rastrup-Andersen, J. (1958)J. Mol. Spectrosc. 2, 361.
Bart, J. C. J. & Daly, J. J. (1970)J. Chem. Soc. (A), 567.
Coggon, P., Engel, J. F., McPhail, A. T. & Quin, L. D. (1970)J. Amer. Chem. Soc. 92, 5779.
Cruickshank, D. W. J. (1956)Acta Crystallogr. 9, 915.
Duchamp. D. J. (1964)Abstr. of the Am. Cryst. Assn., Bozeman, Montana, paperB14, p. 29.
Goetz, H., Juds, H. & Marschner, F. (1972)Phosphorus,1, 217.
Häring, M. (1960)Helv. Chim. Acta,43, 1826.
International Tables for X-ray Crystallography, Vol. III (Kynoch Press, Birmingham, England, 1968a, p. 202; 1968b, p. 276).
Jenkins, R. N., Freedman, L. D. & Bordner, J. (1971),J. Chem. Soc. (D), 1213.
Johnson, C. K. (1965) ORTEP, ORNL-3794, Oak Ridge National Laboratory, Oak Ridge, Tennessee.
Long, R. E. (1965) Ph.D. Thesis, University of California at Los Angeles.
Märkl, G.Chimie Organique du Phosphore (Centre National de la Recherche Scientifique, Paris, France, 1970, pp. 295–300).
Majeste, R. & Trefonas, L. M. (1969)J. Heterocycl. Chem. 6, 269.
Mann, F. G.The Heterocyclic Derivatives of Phosphorus, Arsenic, Antimony and Bismuth, Second Edition (Wiley-Interscience, New York, 1970, pp. 3–354).
Mark, V., Dungan, C. H., Crutchfleld, M. M. & Van Wazer, J. R.Topics in Phosphorus Chemistry, Vol. 5 (Wiley-Interscience, New York, 1967, p. 382).
Sayre, D. (1952)Acta Crystallogr. 5, 60.
Stewart, R. F., Davidson, E. R. & Simpson, W. T. (1965)J. Chem. Phys. 42, 3175.
Williams, J. C., Jr., Kuczkowski, J. A., Portnoy, N. A., Yong, K. S., Wander, J. D. & Aguiar, A. M. (1971)Tetrahedron Lett. 4749.
Wilson, A. J. C. (1942)Nature,150, 152.
Author information
Authors and Affiliations
Additional information
Taken in part from the thesis submitted by R. N. Jenkins for the Ph.D. degree, August 1972.
Rights and permissions
About this article
Cite this article
Jenkins, R.N., Freedman, L.D. & Bordner, J. Crystal structure of a spirophosphonium compound, C24H18ClN2P,CH3OH. Journal of Crystal and Molecular Structure 3, 103–114 (1973). https://doi.org/10.1007/BF01237433
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01237433