Abstract
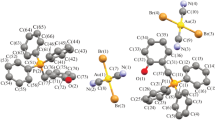
(4-Fluorobenzyl)triphenylphosphonium dicyanodihaloaurates [Ph3PCH2C6H4F-4][Au(CN)2Hlg2], Hlg = Cl, Br, and I, were synthesized from (4-fluorobenzyl)triphenylphosphonium chloride and potassium dicyanodihaloaurate in an aqueous medium. Structure of the compounds was characterized by IR, 1H, 13C{1H}, and 19F{1H} NMR spectroscopy, elemental analysis, and X-ray structural analysis. The crystals of the synthesized complexes contain tetrahedral (4fluorobenzyl)triphenylphosphonium cations and square centrosymmetric [Au(CN)2Hlg2]– anions.





Similar content being viewed by others
REFERENCES
Xiaobo, L. and Patterson, H., Materials, 2013, vol. 6, p. 2595. https://doi.org/10.3390/ma6072595
Dechambenoit, P., Ferlay, S., Kyritsakas, N., and Hosseini, M.W., Cryst. Eng. Commun., 2011, vol. 13, p., 1922. https://doi.org/10.1039/C0CE00607F
Hill, J.A., Thompson, A.L., and Goodwin, A.L., J. Am. Chem. Soc., 2018, vol. 138, p. 5886. https://doi.org/10.1021/jacs.5b13446
Assefaa, Z., Haireb, R.G., and Sykorac, R.E., J. Solid State Chem., 2008, vol. 181, p. 382. https://doi.org/10.1016/j.jssc.2007.11.036
Brown, M.L., Ovens, J.S., and Leznoff, D.B., Dalton Trans., 2017, vol. 46, p. 7169. https://doi.org/10.1039/C7DT00942A
Chorazy, S., Wyczesany, M., and Sieklucka, B., Molecules, 2017, vol. 22, p. 1902. https://doi.org/10.3390/molecules22111902
Shaw, C.F., Chem. Rev., 1999, vol. 99, no. 9, p. 2589. https://doi.org/10.1021/cr980431o
Rawashdeh-Omary, M.A., Omary, M.A., and Patterson, H.H., J. Am. Chem. Soc., 2000, vol. 122, no. 42, p. 10371. https://doi.org/10.1021/ja001545w
Rawashdeh-Omary, M.A., Omary, M.A., Shankle, G.E., and Patterson, H.H., J. Phys. Chem. B, 2000, vol. 104, no. 26, p. 6143. https://doi.org/10.1021/jp000563x
Colis, J.C.F., Larochelle, C., Ferna´ndez, E.J., López-deLuzuriaga, J.M., Monge, M., Laguna, A., Tripp, C., and Patterson, H., J. Phys. Chem. B, 2005, vol. 109, no. 10, p. 4317. https://doi.org/10.1021/jp045868g
Assefaa, Z., Kalachnikova, K., Hairec, R.G., and Sykora, R.E., J. Solid State Chem., 2007, vol. 180, p. 3121. https://doi.org/10.1016/j.jssc.2007.08.032
Roberts, R.J., Le, D., and Leznoff, D.B., Inorg. Chem., 2017, vol. 56, no. 14, p. 7948. https://doi.org/10.1021/acs.inorgchem.7b00735
Ovens, J.S. and Leznoff, D.B., Dalton Trans., 2011, vol. 40, p. 4140. https://doi.org/10.1039/c0dt01772h
Ovens, J.S., Truong, K.N., and Leznof, D.B., Dalton Trans., 2012, vol. 41, p. 1345. https://doi.org/10.1039/c1dt11741f
Ovens, J.S. and Leznoff, D.B., Chem. Mater., 2015, vol. 27, no. 5, p. 1465. https://doi.org/10.1021/cm502998w
Sharutin, V.V., Sharutina, O.K., and Popkova, M.A., Russ. J. Inorg. Chem., 2019, vol. 64. 6, p. 729. https://doi.org/10.1134/S0036023619060147
Sharutin, V.V., Popkova, M.A., and Tarasova, N.M., Bull. South Ural State University, Ser. Chem., 2018, vol. 10, no. 1, p. 55. https://doi.org/10.14529/chem180107
Ovens, J.S., Geisheimer, A.R., Bokov, A.A., Ye, Z.-G., and Leznoff, D.B., Inorg. Chem., 2010, vol. 49, p. 9609. https://doi.org/10.1021/ic101357y
Pitteri, B., Bortoluzzi, M., and Bertolasi, V., Transition Met. Chem., 2008, vol. 33, p. 649. https://doi.org/10.1007/s11243-008-9092-9
Marangoni, G., Pitteri, B., Bertolasi, V., Ferretti, V., and Gilli, G., J. Chem. Soc. Dalton Trans., 1987, no. 1, p. 2235. https://doi.org/10.1039/DT9870002235
Ovens, J.S., Truong, K.N., and Leznoff, D.B., Inorg. Chim. Acta, 2013, vol. 403, p. 127. https://doi.org/10.1016/j.ica.2013.02.011
Senchurin, V.S., Bull. South Ural State University, Ser. Chem., 2019, vol. 11, no. 3, p. 50. https://doi.org/10.14529/chem190306
Sharutin, V.V., Sharutina, O.K., Tarasova, N.M., and Efremov, A.N., Russ. J. Inorg. Chem., 2020, vol. 65, no. 2, p. 169. https://doi.org/10.1134/S0036023620020151
Pretsch, D. P., Bühlmann, P., and Affolter; C., Structure Determination of Organic Compounds, Berlin: Springer Verlag, 2000.
Jones, L., Inorg. Chem., 1964, vol. 3, no. 11, p. 1581. https://doi.org/10.1021/ic50021a024
Shorrock, C.J., Jong, H., Batchelor, R.J., and Leznoff, D.B., Inorg. Chem., 2003, vol. 42, p. 3917. https://doi.org/10.1021/ic034144
Cordero, B., Gómez, V., Platero-Prats, A.E., Revés, M., Echeverría, J., Cremades, E., Barragan, F., and Alvarez, S., Dalton Trans., 2008, p. 2832. https://doi.org/10.1039/b801115j
Mantina, M., Chamberlin, A.C., Valero, R., Cramer, C.J., and Truhlar, D.G., J. Phys. Chem. A, 2009, vol. 113, p. 5806. https://doi.org/10.1021/jp8111556
Bruker (1998). SMART and SAINT-Plus. Versions 5.0. Data Collection and Processing Software for the SMART System. Bruker AXS Inc., Madison, Wisconsin, USA.
Bruker (1998). SHELXTL/PC. Versions 5.10. An Integrated System for Solving, Refining and Displaying Crystal Structures from Diffraction Data. Bruker AXS Inc., Madison, Wisconsin, USA.
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., Howard, J.A. K., and Puschmann, H., J. Appl. Cryst., 2009, vol. 42, p. 339. https://doi.org/10.1107/S0021889808042726
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Additional information
Translated from Zhurnal Obshchei Khimii, 2021, Vol. 91, No. 11, pp. 1716–1722 https://doi.org/10.31857/S0044460X21110081.
Rights and permissions
About this article
Cite this article
Sharutin, V.V., Sharutina, O.K., Tarasova, N.M. et al. Synthesis and Structure of (4Fluorobenzyl)triphenylphosphonium Dicyanodihaloaurates [Ph3PCH2C6H4F-4][Au(CN)2Hlg2]. Russ J Gen Chem 91, 2187–2193 (2021). https://doi.org/10.1134/S1070363221110086
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221110086