Summary
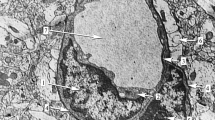
The blood—brain barrier in the cuttlefishSepia officinalis has been studied with the freeze-fracture technique. Previous thin-section electron microscopy showed that a restricting junction is formed between perivascular glial processes in microvessels and venous vessels, and between pericytes in arterial vessels; the restriction appeared not to be a classicalzonula occludens or septate junction. In freeze-fracture replicas from brain optic and vertical lobe, endothelial cells, pericytes and perivascular glia could be recognized by their morphology and relation to the vascular lumen. In microvessels, endothelial and pericyte membranes showed sparse but uniform distribution of P-face intramembranous particles, with no particular particle aggregations. Perivascular glial membranes had a higher density of intramembranous particles but again no particle alignments characteristic of known restricting junctions were seen, although clusterings of intramembranous particles resembling gap junctions were present. In larger venous vessels, the perivascular glial layer showed a multilamellated organization, but again no arrays of intramembranous particles were detected, although this should be a favourable site for visualization of the restricting junctions. The walls of arterial vessels showed collagen deposits and cell processes with apparent intracellular myofilamentous profiles, but no intramembranous junctional particle arrays. It is concluded that the junctional zone observed in thin section electron microscopy is not associated with aligned aggregations of intramembranous particles detectable in freeze-fracture replicas, strengthening the evidence that this is a novel type of restricting junction.
Similar content being viewed by others
References
Abbott, N. J. &Bundgaard, M. (1987) Microvessel surface area, density and dimensions in brain and muscle of the cephalopodSepia officinalis.Proceedings of the Royal Society B 230, 459–82.
Abbott, N. J. &Bundgaard, M. (1992) Electron-dense tracer evidence for a blood—brain barrier in the cuttlefishSepia officinalis.Journal of Neurocytology 21, 276–294.
Abbott, N. J., Bundgaard, M. &Cserr, H. F. (1985a) Brain vascular volume, electrolytes and blood—brain interface in the cuttlefishSepia officinalis (Cephalopoda).Journal of Physiology 368, 197–212.
Abbott, N. J., Bundgaard, M. &Cserr, H. F. (1985b) Tightness of the blood—brain barrier and evidence for brain interstitial fluid flow in the cuttlefishSepia offidnalis.Journal of Physiology 368, 213–26.
Abbott, N. J., Bundgaard, M. &Cserr, H. F. (1986) Comparative physiology of the blood—brain barrier. InThe Blood-Brain Barrier in Health and Disease (edited bySuckling, A. J., Rumsby, M. G. &Bradbury, M. W. B.) pp. 52–72. Chichester: Ellis Horwood.
Abbott, N. J., Bundgaard, M., Lane, N. J. &Mølgård, K. (1988) Parallels between junctions in invertebrate brain and embryonic mammalian brain.Journal of Physiology 400, 72P.
Abbott, N. J., Lane, N. J. &Bundgaard, M. (1992) A fibre-matrix model for the restricting junction of the blood—brain barrier in a cephalopod mollusc: implications for capillary and epithelial permeability.Journal of Neurocytology 21, 304–311.
Arnn, J. &Staehelin, L. A. (1981) The structure and function of spot desmosomes.Dermatology 20, 330–9.
Baldwin, K. M., Loeb, M. J. &Riemann, J. G. (1987) A novel occluding junction which lacks membrane fusion in insect testis.Tissue & Cell 19, 413–21.
Bundgaard, M. &Abbott, N. J. (1992) Fine structure of the blood—brain interface in the cuttlefishSepia officinalis (Mollusca, Cephalopoda).Journal of Neurocytology 21, 260–275.
Cserr, H. F., ed. (1986) The Neuronal Microenvironment. Annals of the New York Academy of Sciences481.
Fain-Maurel, M. A. &Cassier, P. (1972) Une nouveau type de jonctions; les jonctions scalariform. Etude ultrastructurale et cytochimique.Journal of Ultrastructure Research 39, 222–38.
Firth, J. A., Bauman, K. F. &Sibley, C. P. (1983) The intercellular junctions of guinea-pig placental capillaries: a possible structural basis for endothelial solute permeability.Journal of Ultrastructure Research 85, 45–57.
Friend, D. S. &Gilula, N. B. (1972) Variations in tight and gap junctions in mammalian tissues.Journal of Cell Biology 53, 758–76.
Gardner-Medwin, A. R. (1983) Analysis of potassium dynamics in brain tissue.Journal of Physiology 335, 393–426.
Gumbiner, B. (1987) The structure, biochemistry, and assembly of epithelial tight junctions.American Journal of Physiology 253, C749–58.
Lane, N. J. (1979) Freeze-fracture and tracer studies on the intercellular junctions of rectal tissues in insects.Tissue & Cell 11, 481–506.
Lane, N. J. (1981) Invertebrate neuroglia — junctional structure and development.Journal of Experimental Biology 95, 7–33.
Lane, N. J. (1984) A comparison of the construction of intercellular junctions in the CNS of vertebrates and invertebrates.Trends in Neurosciences 7, 95–9.
Lane, N. J. (1989) Novel arthropod cell junctions with restrictive intercellular ‘linkers’.Journal of Neurocytology 18, 661–9.
Noirot-Timothae, C. &Noirot, C. (1980) Septate and scalariform junctions in arthropods.International Review of Cytology 63, 97–140.
Orkand, R. K., Orkand, P. M. &Tang, C. -M. (1981) Membrane properties of neuroglia in the optic nerve ofNecturus.Journal of Experimental Biology 95, 49–59.
Peracchia, C. (1980) Structural correlates of gap junction permeation.International Review of Cytology 66, 81–146.
Peracchoa, C. &Dulhunty, A. F. (1976) Low resistance junctions in crayfish: structural changes with functional uncoupling.Journal of Cell Biology 70, 419–39.
Shaw, S. R. &Henken, D. B. (1984) InInsect Neurochemistry and Neurophysiology (edited byBorkovec, A. B. &Kelley, T. J.) pp. 471–473. New York and London: Plenum Press.
Staehelin, L. A. (1974) Structure and function of intercellular junctions.International Review of Cytology 39, 191–283.
Swales, L. S. &Lane, N. J. (1985) Embryonic development of glial cells and their junctions in the locust CNS.Journal of Neuroscience 5, 117–27.
Villegas, G. M., Lane, N. J. &Villegas, J. (1987) Freeze-fracture studies on the giant axon and ensheathing Schwann cells of the squid.Journal of Neurocytology 16, 11–21.
Zwahlen, M. J., Sandri, C. &Greeff, N. G. (1988) Transglial pathway of diffusion in the Schwann sheath of the squid giant axon.Journal of Neurocytology 17, 145–59.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lane, N.J., Abbott, N.J. Freeze-fracture evidence for a novel restricting junction at the blood—brain barrier of the cuttlefishSepia officinalis . J Neurocytol 21, 295–303 (1992). https://doi.org/10.1007/BF01224762
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01224762