Abstract
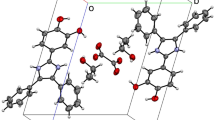
The title compound [Ni(napyo)2(H2O)2](NO3)2 was formed by the reaction of nickel(II) nitrate with 1,8-naphthyridine-N-monoxide (napyo) in ethanol medium. The complex crystallizes in the monoclinic, space groupP21/n withZ=2. Lattice parameters are:a=7.160(2),b=11.713(3),c=11.830(2) Å,β=95.11(3)°. The structure was determined from 1399 observed reflections and refined toR=0.061. The Ni atom shows a slightly distorted octahedral coordination, being bonded to two oxygen atoms and two nitrogen atoms of two napyo ligands and to two oxygen atoms of two water molecules.
Similar content being viewed by others
References
Frenz, B. A., and Okaya, Y. (1980)Enfra-Nonius Structure Determination Package. (Delft, Holland).
Gan, X., Tang, N., Wang, X., Zhu, Y., and Tan, M. (1989)Polyhedron,8, 933–934.
International Tables forX-Ray Crystallography (1974) (Kynoch Press, Birmingham, U.K.), Vol. IV.
Kobayashi, Y., Kumadaki, I., Stao, H., Sekine, Y., and Hara, T. (1974)Chem. Pharm. Bull. 22, 2097–2100.
North, A. C. T., Phillips, D. C., and Mathews, F. S. (1968)Acta Crystallogr. A 24, 351–359.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, X., Gan, X., Tang, N. et al. Preparation and crystal structure of diaquabis(1,8-naphthyridine-N-monoxide)nickel(II)nitrate. Journal of Crystallographic and Spectroscopic Research 20, 515–517 (1990). https://doi.org/10.1007/BF01221890
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01221890