Summary
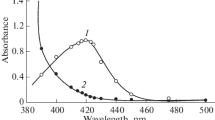
A spectrophotometric study has been made of the solvent extraction of molybdophosphoric acid and its blue reduction product with propylene carbonate from acidic solutions. Molybdophosphoric acid forms an adduct with propylene carbonate which is readily extracted with chloroform. The molar absorptivity of the molybdophosphoric acid in the propylene carbonate-chloroform phase is 22300 1 mole−1 cm−1 at 308 nm. Although experimental conditions for the extraction of the heteroply blue must be more carefully controlled, this extract exhibits a molar absorptivity of 28500 1 mole−1 cm−1 at 790 nm.
Zusammenfassung
Eine spektrophotometrische Untersuchung der Extrahierbarkeit der Molybdänphosphorsäure und ihres blauen Reduktionsproduktes mit Propylencarbonat aus saurer Lösung wurde durchgeführt. Molybdänphosphorsäure bildet mit Propylencarbonat ein Addukt, das mit Chloroform gut extrahierbar ist. Ihre molare Absorption in der Propylencarbonat-Chloroform-Phase beträgt 223001·mol−1 · cm−1 bei 308 nm. Die experimentellen Bedingung für die Extraktion der blauen Heteropolysäuren müssen zwar noch genauer geprüft werden, die molare Extinktion kann jedoch mit 285001·mol−1·cm−1 bei 790 nm angegeben werden.
Similar content being viewed by others
References
T. J. Hastings, Jr., andH. A. Frediani, Analyt. Chemistry20, 382 (1948).
D. N. Bernhart andA. R. Wreath, Analyt. Chemistry27, 440 (1955)
M. A. DeSesa andL. B. Rogers, Analyt. Chemistry26, 1381 (1954)
C. Wadelin andM. G. Mellon, Analyt. Chemistry25, 1668 (1953).
R. A. Chalmers andD. A. Thomson, Analyt. Chim. Acta18, 575 (1958)
D. F. Boltz andM. G. Mellon, Analyt. Chemistry19, 873 (1947).
C. H. Fiske andY. Subbarow, J. Biol. Chem.66, 375 (1925).
R. D. Bell andE. A. Doisy, J. Biol. Chem.44, 55 (1920).
E. W. Scarritt, Ind. Eng. Chem., Analyt. Ed.3, 23 (1931).
S. Byall andJ. A. Ambler, Ind. Eng. Chem., Analyt. Ed.3, 136 (1931)
B. E. Reznik andL. P. Tsyganok, Zh. Anal. Khim.19, 540 (1964)
N. B. Vinogradova et al., Zh. Anal. Khim.19, 925 (1964).
T. Kuttner andH. R. Cohen, J. Biol. Chem.75, 517 (1925).
O. H. Lowry andJ. A. Lopez, J. Biol. Chem.162, 421 (1946).
J. L. Hague andH. A. Bright, J. Res. N. B. S.26, 405 (1941).
S. Burstein, Analyt. Chemistry25, 422 (1953).
J. B. Martin andD. M. Doty, Analyt. Chemistry21, 965 (1949).
G. A. Bauer, Analyt. Chemistry37, 155 (1965).
W. S. Clabaugh andA. Jackson, J. Res. N. B. S.62, 201 (1959).
F. L. Schaffer et al., Analyt. Chemistry25, 343 (1952).
W. A. Pons andJ. D. Guthrie, Ind. Eng. Chem., Analyt. Ed.18, 1 (1946).
J. Paul, Analyt. Chim. Acta35, 200 (1966).
P. Pakalns, Analyt. Chim. Acta40, 1 (1968).
C. Lueck andD. F. Boltz, Analyt. Chemistry28, 1168 (1956).
N. S. Ging, Analyt. Chemistry28, 1330 (1956).
V. Klitina et al., Zh. Anal. Khim.20, 1197 (1965).
J. Paul, Analyt. Chim. Acta23, 178 (1960).
C. P. Sideris, Ind. Eng. Chem., Analyt. Ed.14, 762 (1942).
J. Paul, Mikrochim. Acta [Wien]1965, 830.
V. Klitina et al., Zh. Anal. Khim.21, 186 (1966).
F. P. Sudakov et al., Zh. Anal. Khim.21, 1186 (1966).
L. Ducret andM. Drouillas, Analyt. Chim. Acta21, 297 (1959).
A. K. Babko et al., Zh. Anal. Khim.21, 172 (1966).
L. C. Mokraseh, Analyt. Chemistry33, 432 (1961).
C. Lueck andD. F. Boltz, Analyt. Chemistry30, 183 (1958).
T. R. Hurford andD. F. Boltz, Analyt. Chemistry40, 379 (1968).
D. F. Boltz andC. Lueck, Colorimetrie Determination of Nonmetals. New York: Interscience. 1958.
M. Jean, Chim. analytique37, 125 (1955).
B. G. Stephens andH. A. Suddeth, Analyt. Chemistry39, 1478 (1967).
Jefferson Chemical Company, Inc., Propylene Carbonate, Technical bulletin, Houston, Texas.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jakubiec, R.J., Boltz, D.F. Spectrophotometric study of molybdophosphoric acid methods for phosphorus using propylene carbonate as extractant. Mikrochim Acta 58, 1199–1207 (1970). https://doi.org/10.1007/BF01215955
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01215955