Abstract
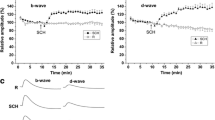
Quisqualic acid, an excitatory amino acid agonist, has been shown to stimulate inositol phosphate production in the rabbit retina. Inositol trisphosphate serves as a second messenger and increases intracellular calcium. We investigated the influence of quisqualic acid on the direct-current electroretinogram and on the standing potential of the rabbit eye. After unilateral vitrectomy, the corneal direct-current electroretinogram and the standing potential were recorded from both eyes of albino rabbits during simultaneous unilateral intravitreal perfusion with quisqualic acid alternating with control solution. The contralateral eye was used as a control. Intravitreal perfusion with 100-µM and 200-µM quisqualic acid elevated the standing potential significantly. This elevation was accompanied by a significant increase in c-wave amplitude and a significant decrease in b-wave amplitude. Quisqalic acid at 200-µM concentration decreased the a-wave amplitude also.In vivo intraretinal recordings showed that intravitreal perfusion with quisqualic acid at 200-µM concentration significantly increased the retinal pigment epithelial component of the c-wave. We conclude that quisqualic acid influences the direct-current electroretinogram and the standing potential apparently through its action on the retinal pigment epithelium. A possible mode of action is increased production of inositol trisphosphate, followed by an increase in intracellular release of calcium ions and an increase in basal chloride conductance. The decrease in a- and b-wave amplitudes indicates direct effects of quisqualic acid also on the neural retina.
Similar content being viewed by others
Abbreviations
- EAA:
-
excitatory amino acid
- IP:
-
inositol phosphate
- NMDA:
-
N-methyl-Daspartate
- PPI:
-
phosphoinositide
- QA:
-
quisqualic acid
References
Morgan IG. Kainic acid as a tool in retinal research. In: Osborne NN, Chader GJ, eds. Progress in retinal research. Oxford: Pergamon Press, 1983; 2: 249–66.
Bloomfield SA, Dowling JE. Roles of aspartate and glutamate in synaptic transmission in rabbit retina I: outer plexiform layer. J Neurophysiol 1985; 53: 699–713.
Massey SC, Redburn DA. Transmitter circuits in the vertebrate retina. Prog Neurobiol 1987; 28: 55–96.
Sladeczek F, Pin JP, Recasens M, Bockaert J, Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurons. Nature 1985; 317: 717–9.
Nicoletti F, Iadarola MJ, Wroblewski JT, Costa E. Excitatory amino acid recognition sites coupled with inositol phospholipid metabolism: Developmental changes and interaction with alpha-l-adrenoreceptors. Proc Natl Acad Sci U S A 1986; 83: 1931–5.
Nicoletti F. Coupling of inositol phospholipid metabolism with excitatory amino acid recognition sites in rat hippocampus. J Neurochem 1986; 46: 40–6.
Recasens M. Characterization of excitatory amino acid receptor subtypes involved in the stimulation of inositol phosphate synthesis in rat brain by synaptoneurosomes. Eur J Pharmacol 1987; 141: 87–93.
Schmidt BH. Dual action of excitatory amino acids on the metabolism of inositol phosphates in striatal neurons. Mol Pharmacol 1987; 32: 364–8.
Recasens M, Guiramand J, Nourigat A, Sassetti I, Devilliers G. A new quisqualate receptor subtype (sAA2) responsible for the glutamate-induced inositol phosphate formation in rat brain by synaptoneurosomes. Neurochem Int 1988; 13: 463–7.
Berridge MJ, Irvine RF. Inositol phosphates and cell signalling, Nature 1989; 341: 197–205.
Osborne NN. Stimulatory and inhibitory actions of excitatory amino acids on inositol phospholipid metabolism in rabbit retina: evidence for a specific quisqualate receptor subtype associated with neurons. Exp Eye Res 1990; 50: 397–405.
Osborne NN, FitzGibbon F, Schwartz G. Muscarinic acetylcholine receptor-mediated phosphoinositide turnover in cultured human retinal pigment epithelium cells. Vision Res 1991; 31: 1119–27.
Feldman EL, Randolph AE, Johnston GC, Del Monte MA, Greene DA. Receptor-coupled phosphoinositide hydrolysis in human retinal pigment epithelium. J Neurochem 1991; 56: 2094–100.
Friedman Z, Delahunty TM, Linden J, Campochiaro PA. Human retinal pigment epithelial cells possess V1 vasopressin receptors. Curr Eye Res 1991; 10: 811–6.
Kuriyama S, Ohuchi T, Yoshimura N, Honda Y. Growth factor-induced cytosolic calcium ion transients in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 1991; 32: 2882–90.
Liu N-P, FitzGibbon F, Nash M, Osborne NN. Epidermal growth factor potentiates the transmitter-induced stimulation of c-AMP and inositol phosphates in human pigment epithelial cells in culture. Exp Eye Res 1992; 55: 489–97.
Osborne NN, FitzGibbon F, Nash M, Liu N, Leslie R, Cholewinski A. Serotonergic, 5-HT2 receptor-mediated phosphoinositide turnover and mobilization of calcium in cultured rat pigment epithelium cells. Vision Res 1993; 33: 2171–9.
Lopez-Colome AM, Fragoso G, Wright CE, Sturman JA. Excitatory amino acid receptors in membranes from cultured human retinal pigment epithelium. Curr Eye Res 1994; 13: 553–60.
Steinberg RH, Linsenmeier RA, Griff ER. Retinal pigment epithelial cell contributions to the electroretinogram and electrooculogram. In: Osborne NN, Chader GJ, eds. Progress in retinal research. Oxford: Pergamon Press, 1985; 4: 33–66.
Witkowsky P, Dudek FE, Ripps H. Slow PIII component of the carp electroretinogram. J Gen Physiol 1975; 65: 119–34.
Karwoski CJ, Proenza LM. Relationship between Müller cell responses, a local transretinal potential, and potassium flux. J Neurophysiol 1977; 40: 244–59.
Karwoski CJ, Proenza LM. Spatio-temporal variables in the relationship of neuronal activity to potassium and glial responses. Vision Res 1981; 21: 1713–8.
Gallemore RP, Steinberg RH. Effects of DIDS on the chick retinal pigment epithelium, II: mechanism of the light peak and other responses originating at the basal membrane. J Neurosci 1989; 9: 1977–84.
Joseph DP, Miller SS. Alpha-l-adrenergic modulation of K and Cl transport in bovine retinal pigment epithelium. J Gen Physiol 1992; 99: 263–90.
Ueda Y, Steinberg RH. Chloride currents in freshly isolated rat retinal pigment epithelial cells. Exp Eye Res 1994; 58: 331–42.
Bialek S, Joseph DP, Miller SS. The delayed basolateral membrane hyperpolarization of the bovine retinal pigment epithelium: mechanism of generation. J Physiol 1995; 484: 53–67.
Brew H, Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature 1987; 327: 707–9.
Amato A, Barbour B, Szatkowski M, Attwell D. Counter-transport of potassium by the glutamate uptake carrier in glial cells isolated from the tiger salamander retina. J Physiol 1994; 479: 371–80.
Schwartz EA. L-glutamate conditionally modulates the K+ current of Müller glial cells. Neuron 1993; 10: 1141–9.
David P, Lusky M, Teichberg VI. Involvement of excitatory neurotransmitters in the damage produced in chick embryo retinas by anoxia and extracellular high potassium. Exp Eye Res 1988; 46: 657–62.
Yoon YH, Marmor MF. Dextromethorphan protects retina against ischemic injuryin vivo. Arch Ophthalmol 1989; 107: 409–11.
Mosinger JL, Price MT, Bai HY, Xiao H, Wozniak DF, Olney JW. Blockade of both NMDA and non-NMDA receptors is required for optimal protection against ischemic neuronal degeneration in thein vivo adult mammalian retina. Exp Neurol 1991; 113: 10–7.
Abu El-Asrar AM, Morse PH, Maimone D, Torczynski E, Reder AT. MK-801 protects retinal neurons from hypoxia and the toxicity of glutamate and aspartate. Invest Ophthalmol Vis Sci 1992; 33: 3463–8.
Weber M, Bonaventure N, Sahel JA. Protective role of excitatory amino acid antagonists in experimental retinal ischemia. Graefes Arch Clin Exp Ophthalmol 1995; 233: 360–5.
Xu X, Xu J, Huang B, Livsey CT, Karwoski CJ. Comparison of pharmacological agents (aspartate vs. aminophosphonobutyric plus kynurenic acids) to block synaptic transmission from retinal photoreceptors in frog. Exp Eye Res 1991; 52: 691–8.
Vaegan, Millar TJ. Effects of kainic acid and NMDA on the pattern electroretinogram, the scotopic threshold response, the oscillatory potentials and the electroretinogram in the urethane anesthetized cat. Vision Res 1994; 34: 1111–25.
Textorius O, Nilsson SEG, Andersson B-E. Effects of intraocular perfusion with two alternating irrigation solutions on the simultaneously recorded electroretinogram of albino rabbits. Doc Ophthalmol 1986; 63: 349–58
Jarkman S. Effects of low doses of forskolin on the c-wave of the direct current electroretinogram and on the standing potential of the eye. Doc Ophthalmol 1988; 67: 305–14.
Jarkman S, Bragadóttir R. Adrenergic effects on the corneal and intraretinal direct-current electroretinogram and on the standing potential of albino rabbit eyes. Doc Ophthalmol 1995; 89: 251–66.
Elenius W. Recovery in the dark of the rabbit's electroretinogram in relation to intensity, duration and colour of light-adaptation. Acta Physiol Scand Suppl 1958; 44: 1–57.
Textorius O, Nilsson SEG. Effects of intraocular irrigation with melatonin on the c-wave of the direct current electroretinogram and on the standing potential of the eye in albino rabbits. Doc Ophthalmol 1987; 65: 97–111.
Jarkman S. Effects of vasoactive intestinal peptide (VIP) on the dc ERG and on the standing potential of albino rabbit eyes, Clin Vision Sci 1992; 7: 71–6.
Schwarcz R, Scholz D, Coyle JT. Structure-activity relations for the neurotoxicity of kainic acid derivatives and glutamate analogues. Neuropharmacology 1978; 17: 145–51.
Hampton CK, Garcia C, Redburn DA. Localization of kainic acid-sensitive cells in mammalian retina. J Neurosci Res 1981; 6: 99–111.
Shimazaki H, Karwoski CJ, Proenza LM. Aspartate-induced dissociation of proximal from distal retinal activity in the mudpuppy. Vision Res 1984; 24: 587–95.
Sarantis M, Everett K, Attwell D. A presynaptic action of glutamate at the cone output synapse. Nature 1988; 332: 451–3.
Steinberg RH, Schmith R, Brown KT. Intracellular responses to light from cat pigment epithelium: origin of the electroretinogram c-wave. Nature 1970; 227: 728–30.
Oakley B II, Green DG. Correlation of light-induced changes in retinal extracellular potassium concentration with c-wave of the electreretinogram. J Neurophysiol 1976; 39: 1117–33.
Noell WK. Studies on the electrophysiology and the metabolism of the retina. Randolph Field, Texas: US Air Force, 1953. SAM Project 21-1201-004.
Miller SS, Steinberg RH. Passive ionic properties of frog retinal pigment epithelium. J Membr Biol 1977; 36: 337–72.
Gallemore RP, Steinberg RH. Effects of DIDS on the chick retinal pigment epithelium, I: membrane potentials, apparent resistances, and mechanisms. J Neurosci 1989; 9: 1968–76.
Miller SS, Edelman JL. Active ion transport pathways in the bovine retinal pigment epithelium. J Physiol 1990; 424: 283–300.
Joseph DP, Miller SS. Apical and basal membrane ion transport mechanisms in bovine retinal pigment epithelium. J Physiol 1991; 435: 439–63.
Fujii S, Gallemore RP, Hughes BA, Steinberg RH. Direct evidence for a basolateral membrane Cl− conductance in toad retinal pigment epithelium. Am J Physiol 1992; 262: C374–83.
Greenberger LM, Besharse JC. Stimulation of photoreceptor disc shedding and, pigment epithelial phagocytosis by glutamate, aspartate, and other amino acids. J Comp Neurol 1985; 239: 361–72.
Besharse JC, Spratt G. Excitatory amino acids and rod photoreceptor disc shedding: analysis using specific agonist. Exp Eye Res 1988; 47: 609–20.
Heth CA, Marescalchi PA. Inositol triphosphate generation in cultured rat retinal pigment epithelium. Invest Ophthalmol Vis Sci 1994; 35: 409–16.
Mawer ML, Miller RJ. Excitatory amino acid receptors, second messengers and regulation of intracellular Ca2+ in mammalian neurons. Trends Pharmacol Sci 1990; 11: 254–60.
Malinow R, Madison DV, Tsien RW. Persistent protein kinase activity underlying longterm potentiation. Nature 1988; 335: 820–4.
Manev H, Favaron M, Guidotti A, Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol 1989; 36: 106–12.
Aniksztejn L, Bregestovski P, Ben-Ari Y. Selective activation of quisqualate metabotropic receptor potentiates NMDA but not AMPA responses. Eur J Pharmacol 1991; 205: 327–8.
Bleakman D, Rusin KI, Chard PS, Glaum SR, Miller RJ. Metabotropic glutamate receptors potentiate ionotropic glutamate responses in the rat dorsal horn. Mol Pharmacol 1992; 42: 192–6.
Schoepp DD, Conn PJ. Metabotropic glutamate receptors in brain function and pathology. Trends Pharmacol Sci 1993; 14; 13–20.
Dixon DB, Copenhagen DR. Two types of glutamate receptors differentially excite amacrine cells in the tiger salamander retina. J Physiol 1992; 449: 589–606.
Witkovsky P, Dearry A. Functional roles of dopamine in the vertebrate retina. In: Osborne NN, Chader GJ, eds. Progress in retinal research. Oxford: Pergamon Press, 1991; 11: 247–92.
Dawis SM, Niemeyer G. Similarity and diversity of monoamines in their effects on the standing potential, light peak and electroretinogram of the perfused cat eye. Clin Vision Sci 1988; 3: 109–18.
Gallemore RP, Steinberg RH. Effects of dopamine on the chick retinal pigment epithelium. Invest Ophthalmol Vis Sci 1990; 31: 67–80.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kato, M., Bragadóttir, R., Jarkman, S. et al. Effects of quisqualic acid on the corneal and intraretinal direct-current electroretinogram and on the standing potential of the rabbit eye. Doc Ophthalmol 91, 349–362 (1995). https://doi.org/10.1007/BF01214653
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01214653