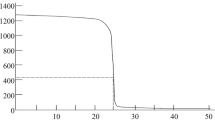
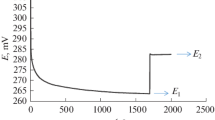
Summary
Ascorbic acid has been determined in fruits and pharmaceuticals by titration with thallium (III) sulfate using promethazine or 4-me-thoxychrysoidine as visual indicators or by potentiometry. Interference of cysteine, glutathione and hydrogensulfite is avoided by their masking with acrylonitrile. Use of cysteine or hydrogensulfite is recommended as an antioxidant during the extraction of ascorbic acid from plant materials.
Zusammenfassung
Die potentiometrische Titration von Ascorbinsäure in Früchten und Arzneimitteln mit Thallium(III) läßt sich unter Verwendung von Promethazin oder 4-Methoxychrysoidin als Indikator durchführen. Die Störung durch Cystein, Glutathion und Hydrogensulfit läßt sich durch Maskierung mit Acrylnitril verhindern. Cystein oder Hydrogensulfit dienen als Antioxydantien bei der Extraktion der Ascorbinsäure aus pflanzlichem Material.
Similar content being viewed by others
References
L. Erdey and E. Bodor, Analyt. Chemistry24, 418 (1952).
G. S. Sastri and G. G. Rao, Talanta19, 212 (1972).
M. Z. Barakat, M. F. Abdel-Wahab, and M. M. El-Sadr, Analyt. Chemistry27, 536 (1955).
O. A. Bessey and C. C. King, J. Biol. Chem.103, 687 (1933).
D. C. Garratt, The Quantitative Analysis of Drugs. London: Chapman & Hall. 1964. p. 96.
D. Gupta, P. D. Sharma, and Y. K. Gupta, Talanta22, 913 (1975).
I. M. Kolthoff and R. Belcher, Volumetric Analysis. Vol. III. New York: Interscience. 1957. p. 370.
L. Hellerman, F. P. Chinard, and P. A. Ramsdell, J. Amer. Chem. Soc.63, 2551 (1941).
P. C. Dwivedi, K. Gurudath, S. N. Bhat, and C. N. R. Rao, Spectrochim. ActaA31, 129 (1975).
H. S. Gowda and S. A. Ahmed, Analyt. Chim. Acta99, 343 (1978).
H. S. Gowda and S. A. Ahmed, Analyst103, 1148 (1978).
G. S. Misra and R. S. Asthana, J. Pract. Chem.4, 270 (1957).
M. Wronski, Analyst85, 526 (1960).
A. Berka, J. Vulterin, and J. Zyka, Newer Redox Titrants. Oxford: Pergamon Press. 1965. p. 163.
S. Bose, M. P. Sahasrabuddhey, and K. K. Verma, Talanta23, 725 (1976).
N. Nazar, M. Y. Ikram-ul-Haq, and A. F. M. Ehteshamuddin, Pakistan J. Sci. Ind. Res.11, 381 (1968).
L. W. Mapson, J. Soc. Chem. Ind.62, 223 (1943).
M. Sarwar, Z. Iqbal, and S. Zaidi, Mikrochim. Acta [Wien]1975, 699.
T. S. Ma and R. E. Lang, Quantitative Analysis of Organic Mixtures. New York: Wiley. 1979.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Verma, K.K., Palod, S. Determination of ascorbic acid in fruits and pharmaceuticals by titration with thallium(III). Mikrochim Acta 80, 361–367 (1983). https://doi.org/10.1007/BF01202013
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01202013