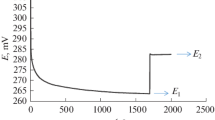
A coordination compound of iron(III) ion with 1,10-phenanthroline, or the oxidized form of ferroin (OFF), which was obtained via the reaction of phenanthroline hydrochloride and iron(III) ammonium sulfate (3:1) in acidic solution and standardized by indirect iodometry, was proposed for use as a titrant for quantitative determination of ascorbic acid (AA) in drugs. The developed method for determining AAwas characterized by satisfactory validation indicators of specificity, linearity (r = 0.990), extended analytical range (50 – 170% AA), accuracy (AA recovery 99.6%), precision (RSD 1.3%), and low relative error of determination (å 2.9%). The procedure for AA analysis using OFF did not depend on the solution pH and ionic strength and enabled titrations in colored and cloudy solutions. The technique was tested on various commercial drugs: 5% AAsolution for injection, Revit drops, AA tablets with glucose, and cinnamon rose hips.



Similar content being viewed by others
References
State Pharmacopoeia of the Russian Federation, XIVth Ed., PM. 2.1.0058.18 Ascorbic Acid, PM. 2.5.0106.18 Rose Hips, Ministry of Health of the Russian Federation, Moscow (2018).
State Pharmacopoeia of the Russian Federation, XIVth Ed., GPM. 1.1.0012.15 Validation of Analytical Methods, GPM. 1.2.1.19.0002.15 Potentiometric Titration, GPM. 1.2.3.0017.15 Quantitative Determination Methods for Vitamins, GPM. 1.3.0001.15 Reagents. Indicators, GPM. 1.3.0002.15 Titration of Solutions, Ministry of Health of the Russian Federation, Moscow (2018).
V. G. Belikov, Pharmaceutical Chemistry [in Russian], Vysshaya Shkola, Moscow (1985), pp. 133, 605 – 609.
S. P. Gladkikh, Khim.-farm. Zh., 4(12), 37 – 42 (1970).
E. G. Vlasova, O. M. Petrukhin, et al., Analytical Chemistry: Chemical Methods of Analysis [in Russian], L. B. Kuznetsova (ed.), Laboratoriya Znanii, Moscow (2021), pp. 379 – 382.
Yu. Ya. Kharitonov, Analytical Chemistry (Analytics) [in Russian], in 2 Vols., Book 1, Vysshaya Shkola, Moscow (2005), pp. 146 – 171.
O. K. Tikhonova, L. A. Drygunova, et al., Analytical Chemistry. Quantitative Chemical Analysis [in Russian], O. K. Tikhonova (ed.), 2nd Ed., SibGMU, Tomsk (2015), pp. 117 – 134.
V. D. Ponomarev, Analytical Chemistry [in Russian], Vysshaya Shkola, Moscow (1985), Part 1, pp. 30 – 34, 85 – 109; Part 2, pp. 105 – 116.
Yu. Yu. Lur’e, Handbook for Analytical Chemistry, Reference Edition [in Russian], 6th Ed., Revised and Supplemented, Khimiya, Moscow (2013), pp. 326 – 335.
State Drug Registry, Moscow (2020); https: //grls.rosminzdrav.ru.
S. N. Shcherbak, V. A. Kompantsev, and N. Sh. Kaisheva, Drug Quality Control (Information-Methodical Bulletin No. 2) [in Russian], in: Materials of the All-Russian Scientific Society of Pharmacists, pp. 107 – 108 (1992).
N. Sh. Kaisheva, A. Sh. Kaishev, and G. V. Smolenskaya, Scientific Review. Basic and Applied Research [in Russian], 2 (2019); https: //scientificreview.ru.
N. Sh. Kaisheva, A. Sh. Kaishev, et al., International Conference on Production and Processing of Agricultural Raw Materials - Technology of Sugars, Saccharine Products and Alcohol, Sept. 22 – 24, 2021, Voronezh, Russian Federation, Materials IOP Conference Earth and Environmental Science, Vol. 1052, p. 012097 (2022).
S. N. Shcherbak, N. Sh. Kaisheva, and V. A. Kompanets, RU Pat. 2,160,100, Dec. 10, 2000; Byull. Izobret., Dec. 10, 2000.
N. Sh. Kaisheva and A. Sh. Kaishev, RU Pat. 2,696,865, Jul. 8, 2019; Byull. Izobret., No. 22 (2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 57, No. 9, pp. 56 – 64, September, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaisheva, N.S., Kaishev, A.S. Development of a Quantitative Determination Technique for Ascorbic Acid in Medicines. Pharm Chem J 57, 1511–1519 (2023). https://doi.org/10.1007/s11094-023-03018-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-03018-5