Abstract
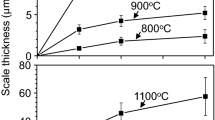
Co−15 at.% Nb alloys containing up to 15 at.% Al were corroded in gaseous H2−H2O−H2S mixtures over the temperature range of 600–900°C. The corrosion kinetics followed the parabolic rate law at all temperatures. Corrosion resistance improved with increasing Al content except at 900°C. Duplex scales formed on alloys consisting of an outer layer of cobalt sulfide and a heterophasic inner layer. A small amount of Al2O3 was found only on Co−15Nb−15Al. Contrary to what formed in Co−Nb binary alloys, neither NbS2 nor NbO2 were found in the inner layer of all alloys, but Nb3S4 did form. The absence of NbS2 and NbO2 is due to the formation of stable Al2O3 and Al2S3 that effectively blocked the inward diffusion of oxygen and sulfur, respectively, and to the reduction of activity of Nb by Al additions in the alloys. Intercalation of ions in the empty hexagonal channels of Nb3S4 is associated with the blockage of the transport of cobalt. An unknown phase (possibly Al0.5NbS2) was detected. Alloys corroded at 900°C were abnormally fast and formed a scale containing CoNb3S6 and Co. Pt markers were found at the interface between the inner and outer layers.
Similar content being viewed by others
References
K. Natesan,Corrosion 41, 646 (1985).
S. Mrowec and K. Przybylski,High Temp. Mater. Proc.,6, 1 (1984).
H. S. Hsu,Oxid. Met. 28, 213 (1987).
K. N. Strafford and P. K. Datta,Mater. Sci. Tech. 5, 765 (1989).
W. Kai, D. L. Douglass, and F. Gesmundo,Oxid. Met. 37, 189 (1992).
C. C. Shing, D. L. Douglass, and F. Gesmundo,Oxid. Met. 37, 167 (1992).
Y. R. He, D. L. Douglass, and F. Gesmundo,Oxid. Met. 37, 217 (1992).
W. Kai, D. L. Douglass, and F. Gesmundo,Oxid. Met. 37, 389 (1992).
C. C. Shing, D. L. Douglass, and F. Gesmundo,Oxid. Met. 37, 441 (1992).
Y. R. He, D. L. Douglass, and F. Gesmundo,Oxid. Met. 37, 413 (1992).
C. C. Shing and D. L. Douglass,Oxid. Met. 40, 155 (1993).
G. Wang, D. L. Douglass, and F. Gesmundo,Oxid. Met. 35, 279 (1991).
V. L. Matukhin, I. A. Safin, V. F. Shamrai, and G. M. Leitus,Sov. Phys. Solid State 27, 117 (1985).
JANAF Thermodynamical Tables, 3rd ed., 1985 supplement, fromJ. Phys. Chem. Ref. Data14 (1985).
W. Kai and D. L. Douglass,Oxid. Met. Oxid. Met. 39, 415 (1993).
N. A. Gokcen,Statistical Thermodynamics of Alloys (Plenum Press, New York, 1986).
L. Kaufman and H. Nesor,Met. Trans. 6A, 2115 (1975).
L. Kaufman and H. Nesor,Calphad 2, 325 (1978).
A. F. J. Ruysink, F. Kadijk, A. J. Wagner, and F. Jellinek,Acta. Crystallogr. Sect. B 24, 1614 (1968).
G. Huan and M. Greenblatt,Mater. Res. Bull. 22, 943 (1987).
R. Schöllhorn and W. Schramm,Z. Naturforsch. Teil B 34, 697 (1979).
B. Gleeson, D. L. Douglass, and F. Gesmundo,Oxid. Met. 31, 209 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shing, C.C., Douglass, D.L. Effect of Al on high-temperature corrosion of Co-15a/oNb alloys in H2-H2O-H2S environments. Oxid Met 41, 115–138 (1994). https://doi.org/10.1007/BF01196646
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01196646