Summary
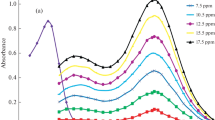
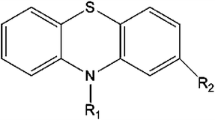
Diethazine hydrochloride forms an yellowish-green complex with platinum(IV) in sodium acetate-hydrochloric acid buffer containing copper catalyst. The complex has an absorption maximum at 404nm and its molar absorptivity is 1.083×104l mole−1cm−1. The sensitivity is 18 ng/cm2 for logI 0/I=0.001. Beer's law is valid over the concentration range 0.5–12.0 ppm. The composition of the complex as determined by the continuous variations, mole ratio and slope ratio methods is 1∶1 and the apparent stability constant of the complex at pH 2.3 and 27° has the log K value of 5.83. The effects due to pH, reagent concentration, copper concentration, time and diverse ions are reported.
Zusammenfassung
10-(2-Diäthylaminoäthyl)-phenothiazinhydrochlorid (DH) bildet mit Platin in Natriumacetat-Salzsäure-Puffer, der Kupfer als Katalysator enthält, eine gelbgrüne Komplexverbindung. Diese hat ein Absorptionsmaximum bei 404 nm, ihre molare Extinktion beträgt 1,083×104l·mol−1·cm−1. Die Empfindlichkeit entspricht 18 ng/cm2 für logI 0/I=0,001. Das Beersche Gesetz gilt zwischen 0,5 und 12,0 ppm. Der Komplex ist im Verhältnis 1∶1 zusammengesetzt. Die scheinbare Stabilitätskonstante bei pH 2,3 und 27° C entspricht logK=5,83. Über den Einfluß von pH, Reagenskonzentration, Kupferkonzentration, Zeit und verschiedene Fremdionen wurde berichtet.
Similar content being viewed by others
References
F. E. Beamish and J. C. Van Loon, Recent Advances in the Analytical Chemistry of the Noble Metals, Oxford: Pergamon. 1972. p. 375.
C. S. Bhandari, M. R. Bhandari, and N. C. Sogani, J. Proc. Instn. Chem. India41, 254 (1969).
A. V. Radushev, A. N. Chechneva, and E. G. Kovalev, Zh. Anal. Khim.23, 1410 (1968).
G. H. Ayres and H. P. Janota, Analyt. Chemistry31, 1985 (1959).
F. E. Piercy and D. E. Ryan, Can. J. Chem.41, 667 (1963).
J. J. Kirkland and J. H. Yoe, Analyt. Chemistry26, 1340 (1954).
J. G. Sen Gupta, Analyt. Chim. Acta23, 462 (1960).
G. H. Ayres and A. S. Meyer, Analyt. Chemistry23, 299 (1951).
A. K. Majumdar and J. G. Sen Gupta, Z. analyt. Chem.177, 265 (1960).
A. Ringbom, Z. analyt. Chem.115, 332 (1938).
G. H. Ayres, Analyt. Chemistry21, 652 (1949).
P. Job, Ann. chim.9, 113 (1928).
H. Irving and T. B. Pierce, J. Chem. Soc. (London)1959, 2565.
J. H. Yoe and A. L. Jones, Ind. Eng. Chem., Analyt. Ed.16, 111 (1944).
A. E. Harvey and D. L. Manning, J. Amer. Chem. Soc.72, 4488 (1950);74, 4744 (1952).
E. E. Rakovskii and B. S. Rabinovich, Isv. sib. Otdel, Akad. Nauk SSSR 1970 (9). Ser. khim. Nauk. (4). 98; Referat Zh., Khim. 19 GD, 1971 (5): Abstr. No. 5G28.
S. K. Sandhwani and P. R. Singh, Talanta20, 248 (1973).
V. M. Ivanov, V. N. Pigurovskaya, and A. I. Busev, Zavod. Lab.38, 1311 (1972).
V. P. Kerentseva, M. D. Lipanova, and L. I. Mas'ko, Z. analyt. Chem.27, 1591 (1972).
E. G. R. Ardagh, F. S. Seaborne, and N. S. Grant, Can. Chem. Met.8, 117, 140 (1924).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gowda, H.S., Jagadeesh, K.S. Spectrophotometric determination of platinum using the diethazine hydrochloride complex. Mikrochim Acta 71, 183–190 (1979). https://doi.org/10.1007/BF01196404
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01196404