Abstract
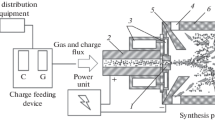
Up to now, calcium carbide has been produced on an industrial scale exclusively by the electrothermal method. Very high-temperature operation should be used, and this results in high capital, and some serious environmental problems if open or half-covered furnaces are used. A chemical equilibrium calculation conducted in this laboratory shows that if we add a certain amount of argon into the precursors, the required temperature for chemical equilibrium of the system can be reduced to 1400–1500 °C. We have been guided to develop a d.c. plasma-heated fluidized bed for the preparation of calcium carbide. Preliminary experiments include cold fluidization, measurement of heat-transfer coefficient, production of calcium carbide and measurement of conversion rate. It was found that 84.3% conversion is reached in an argon atmosphere under atmospheric pressure and an operation temperature of 1400–1450 °C. X-ray diffraction analysis and SEM show that the generated calcium carbide is of good quality.
Similar content being viewed by others
References
Helmut Von Zeppelin, Molhin, Heinz Kalhammer, Waldshut andKlaus Hohmann, Swiss Pat. 437 228 (1967).
M. Frankl, US Pat. 2131 102 (1938).
Bois Eastman, US Pat. 3 017 259 (1920).
Bruce H. Sage, US Pat. 3 044 858 (1962).
N. V. Stamicarbon, Br. Pat. 863 191 (1959).
Idem, Br. Pat. 737 857 (1955).
Kes Van Loon, US Pat. 2 814 478 (1957).
“Ullmann's Encyclopedia of Industrial Chemistry”, Vol. 4 (VCH, Deerfield Beach, USA, 1985).
S. Kiritani andT. Nishimaki,Chem. Economy Eng. Rev. 16(5) (1984) 27.
S. Morooka, T. Okubo andK. Kusakabe,Powd. Technol. 63 (1990) 105.
T. Okubo, H. Kawamura, K. Kusakabe andS. Morooka,J. Am. Ceram. Soc. 73 (1991) 1150.
H. Kawamura,J. Mater. Sci. Lett. 9 (1990) 1033.
M. Nikravech, L. Outifa andJ. E. Amouroux, “Plasma-fluidized bed hydrocracking process of heavy hydrocarbons”, ISPC-9 Japan (1989).
Ph. Arnould, S. Cavadias, J. Amouroux andP. H. Arnould, “The interaction of a fluidized bed with a thermal plasma. Application to the limestone decomposition”, 7th International Symposium of Plasma Chemistry, Eindhoven, July 1985.
A. N. Efimov, M. I. Zhikharev andYu. P. Zhirnov,At. Energ. 39 (1975) 416.
S. Gorden andB. J. McBride, NASA SP-237 (1976).
D. R. Stull andH. Prophet (eds), “JANAF Thermochemical Tables”, 2nd Edn (1971).
C. W. Zhu, G. Y. Zhao andV. Hlavacek,J. Mater. Sci. 27 (1992) 2211.
R. R. Pattipati andC. Y. Wen,Ind. Eng. Chem. Process Des. Dev. 20 (1981) 705.
Herold Y. Atwell andN. Y. Fishkill, US. Pat. 3 017 244 (1962).
H. S. Mickley,A.I.Ch.E.J. September (1955).
J. S. M. Botterill,A.I.Ch.E. Symp. Ser. 208 77 (1881) 330.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhu, C.W., Zhao, G.Y. & Hlavacek, V. A d.c. plasma-fluidized bed reactor for the production of calcium carbide. J Mater Sci 30, 2412–2419 (1995). https://doi.org/10.1007/BF01184594
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01184594