Abstract
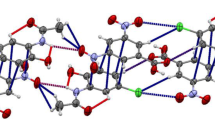
The crystal structure of [Zn(O2CC(CH3)CHCH3)0.54(O2CC6H5)1.46]x has been determined by single crystal X-ray diffraction. The compound crystallizes in the monoclinic system, space group P21/c witha=10.311(1),b=13.170(1),c=19.000(2) Å,β=91.895(7)°,V=2578.7 Å3,Z=4,D x=1.525g cm−3, λ(Mo Kα)=0.71073 Å,μ=1–95 mm−1, F(000)=1206,T=295K,R=0.046 for 4548 unique reflections. The two different carboxylates are randomly scrambled along a one dimensional polymer, made up of binuclear units connected by a syn-anti carboxylate bridge. Each binuclear unit contains three syn-syn carboxylates which bridge two zinc atoms. Vibrational data indicate that [Zn(O2CC(CH3)CHCH3)2]x has the same polymeric structure; orientation of tiglate is the same as for the mixed ligand compound. Recrystallization of mixed carboxylate species produces either an alteration in carboxylate ligand ratio, or a total separation of the two carboxylates, giving two different compounds.
Similar content being viewed by others
References
Booth, C. A., Foxman, B. M., and Jaufmann, J. D. (1987) ACS Symposium Series No. 337, Ch. 7, Crystallographically Ordered Polymers. Sandman, D. J. (ed.).
Bryant, R. G. Chacko, V. P., and Etter, M. C. (1984)Inorg. Chem. 23, 3580.
Clegg, W. (1981)Acta Crystallogr. A 37, 22.
Clegg, W., Little, I. R., and Straughan, B. P. (1986)Acta Crystallogr. C 42, 919.
Cohen, M. D., and Schmidt, G. M. J. (1964)J. Chem. Soc. 1966.
Guseinov, G. A., Musaev, F. N., Usubaliev, B. T., Amiraslanov, I. R., and Mamedov, Kh. S. (1984)Koord. Khim. 10, 117.
Hirshfeld, F. L., and Schmidt, G. M. J. (1964)J. Polym. Sci. A 2, 2181.
International Tables for X-ray Crystallography (1974), Vol. 4 (Kynoch Press, Birmingham), pp. 99, 149.
Mehrotra, R. C., and Bohra, R. (1983)Metal Carboxylates (Academic Press, New York).
Naruchi, K., and Miura, M. (1987)J. Chem. Soc. Perkin Trans. II, 113.
Oldham, C. (1968)Prog. Inorg. Chem. 10, 223.
Restaino, A. J., Mesrobian, R. B. Morawetz, H., Ballantine, D. S., Dienes, G. J., and Metz, D. J. (1956)J. Am. Chem. Soc. 78, 2939.
Sheldrick, G. M. (1985)Shelxtl: An integrated system for solving, refining and displaying crystal structures from diffraction data (University of Göttingen, revision 5).
Shimizu, S., Kekka, S., Kashino, S., and Haisa, M. (1974)Bull. Chem. Soc. Jpn. 47, 1627.
Wang, H., and Robertson, B. E. (1985)Structure and Statistics in Crystallography, ed., Wilson, A. J. C. (Adenine Press. New York), p. 125.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Clegg, W., Harbron, D.R. & Straughan, B.P. Polymeric and mixed carboxylate compounds: Crystal structure of [Zn(tiglate)0.54 (benzoate)1.46]x . Journal of Crystallographic and Spectroscopic Research 20, 17–22 (1990). https://doi.org/10.1007/BF01181670
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01181670