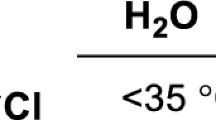Summary
-
1.
In the condensation of malonic acid with acetaldehyde-ammonia, it was found possible to increase the yield of β-aminobutyric acid, which was isolated in the form of its benzoyl derivative, up to the value of 36%, if a catalyst (trimethylphenylammonium hydroxide) was used.
-
2.
The benzoyl group does not split off from β-benzamidobutyric acid when the latter is boiled for 6 hr with 20% HCl, or with 10% KOH, or with glacial acetic acid containing HCl.
-
3.
As in the case of other β-amino acids studied previously, in the preparation of the amide of β-benzamidobutyric acid there is formed also the corresponding methylloxophenyltetrahydropyrimidine. The yield of the latter depends on the amound of thionyl chloride taken in the reaction of acid chloride formation and on the temperature at which this reaction is carried out.
-
4.
When treated with hypobromite, β-benzamidobutyramide gives methylimidazolidone. Under the conditions of the reaction and of the subsequent treatment, the latter is partially hydrolyzed and is converted into propylenediamine.
Similar content being viewed by others
Literature cited
Engel. Ber., 21, 523 (1888); Comptes rendues, 106, 1677 (1888).
Balbiano. Gaz.chim.Ital., 10, 139 (1880); Ber., 13, 312 (1880).
Weidel, Reitner. M. 17, 186 (1896).
E.Fischer. Ber., 34, 343, 3751 (1901).
T.Curtius. J. prak. Chem., (2) 70, 209 (1904).
E. Fischer, H. Scheibler. Ann., 383, 337 (1911).
H. Scheibler, Ber., 45, 2278 (1912).
Skita, Wulff. ann., 453, 206 (1927).
Sienberg. Z. klin. Med., 38, 78 (1899).
K. Morsch. M. 60, 50 (1952).
V.M.Rodionov and E.I.Malevinskaya, Ber. 59, 2952 (1928).
V.M. Rodionov and A.M.Fedorova. Arch. der. Phar., 1933 p. 287; Ber., 60, 804 (1927).
V.M. Rodionov and V.K.Zvorykins. Bull. acad. Sci. USSR, Div. Chem. Sci., 3, 216 (1943).
V.M.Rodionov and V.V.Kiseleva. J. Gen. Chem., 18, 1913 (1948).
E.Fischer, Roder. Ber., 34, 3751 (1901).
Kreibsbakh. Thesis, Moscow State Univ., Medical Faculty. 1906.
Engel. Bull. Soc. chim. France, 50, 102 (1888).
V.M.Rodionov and V.K.Zvorykina, Proc. Acad. Sci. USSR, 45, No. 6, 853 (1949).
F.Lippich. Ber., 41, 2958, 2974 (1908).
K. Lange and F. Adickes. Z. physiol. Chem., 269, 263 (1941).
P. Karrer and A.Schlosser. Helv. Acta, 6, 411 (1932).
S.I.Kanevskaya. J. prak. Chem., (2) 132, 355 (1932).
V.K.Zvorykina. Thesis. Moscow, 1944.
V.M.Rodionov. and v.V.Kiseleva. J. Gen. Chem., 18, 1905 (1948).
L. Gattermann and T. Wieland. Laboratory Methods of Organic Chemistry. State Chemical Press, 1948, p. 245.
V.M.Rodionov and N.G.Yartseva. Bull. acad. Sci. USSR, Div. Chem. Sci., 2, 251 (1948).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rodionov, V.M., Yartseva, N.G. Investigations relating to β-amino acids synthesis and reactions of β-aminobutyric acid. Russ Chem Bull 1, 113–122 (1952). https://doi.org/10.1007/BF01176579
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01176579