Summary
-
1.
Experimental data have been obtained with the object of elucidating the dependence of the extent of isotopic exchange of bromine in K2[PtBr4] and K2[PtBr6] (aqueous solutions) on the duration of the exchange reaction, the concentration of the complex ion, the concentration of bromide ions, the temperature, and the presence of sunlight. An exchange-acceleration effect was detected in aqueous solutions of K2[PtBr4] that had been allowed to stand.
-
2.
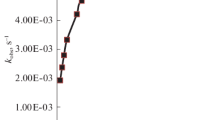
On the basis of the data obtained, tentative calculations were made of the activation energies of the exchange process in the [PtBr4]−− and [PtBr6]−− ions. Under the conditions studied the activation energy for bromine exchange in the [PtBr6]−− ion (4–10 kcal/mole) was found to be appreciably less than the activation energy for the [PtBr4]−− ion (about 17 kcal/mole).
-
3.
On the basis of the data obtained it is concluded that the mechanism of the exchange reaction is different in the [PtBr4]−− and [PtBr4]−− ions.
-
4.
Over the concentration range studied, exchange with K2[PtBr4] in aqueous solution proceeds to a large extent though the intermediate formation of aquo ions.
-
5.
The character of the dependence of the degree of exchange on the bromide ion concentration, the values obtained for the activation energy, the effect of sunlight on the degree of exchange, and also some previously studied properties of quadrivalent platinum derivatives enable us to postulate that exchange with K2[PtBr6] proceeds mainly via an oxidation-reduction equilibrium.
-
6.
The data obtained in this work for the [PtBr4]−− and [PtBr6]−− ions again confirm the conclusion first reached by Grinberg and Nikolskaya, namely, that the instability of a complex ion, as measured by its instability constant, by no means always determines the rate at which it undergoes exchange.
Similar content being viewed by others
Literature cited
A. A. Grinberg and F. M. Filinov, Proc. Acad. Sci. USSR 623, 912 (1939).
A. W. Adamson, I. P. Welker, W. B. Wright, J. Am. Chem. Soc., 73, 3786 (1951).
A. A. Grinberg, L. I. Kozlova, L. E. Nikolskaya and G. A. Shagisultanova, J. Appl. Chem. 28, No. 1, 1955 (T.p. 5).
A. W. Adamson and R. G. Wilkins, J. Amer. Chem. Soc., 76, 3379 (1954).
R. Rich, H. Taube, J. Am. Chem. Soc., 76 2698 (1954).
A. A. Grinberg and L. E. Nikolskaya, J. Appl. Chem. 22, 542 (1949); J. Appl. Chem. 9, 893 (1951). (T.p. 1027)
A. A. Grinberg, B. V. Ptitsyn, and V. N. Lavrentyev, Proc. Acad. Sci., USSR, 26, 51 (1940).
A. A. Grinberg and F. M. Filinov, Bull. Acad. Sci. USSR, Div. Chim. Sci., 1245 (1937).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grinberg, A.A., Shagisultanova, G.A. Kinetics of bromine exchange in complex platinum bromides. Russ Chem Bull 4, 895–900 (1955). https://doi.org/10.1007/BF01172105
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01172105