Abstract
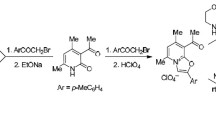
The reaction of 2-X 6-methylpyridines (X = Cl, Br, OMe, OH, ONa) with phenacyl bromides has been studied. It was shown that for X = Cl or Br the reaction products unexpectedly proved to be the previously unknown 5-methyl homologs of oxazolo[3,2-alpyridinium, the structure of which was confirmed by spectral data and by an alternate synthesis using the acid cyclization of N-phenacyl-6-methylpyrid-2-one. The model compound required for the alternate synthesis was obtained by the phenacylation of 2-methoxy-6-methyl-pyridine, 6-methylpyrid-2-one, and its sodium salt. Competition between N- and O-alkylation was observed in the last two cases. The structures of the N- and O-isomers were assigned on the basis of spectral data and by comparison with the spectra of the lower homologs.
Similar content being viewed by others
References
A. E. Tschitschibabin, Chem. Ber.,59, 2084 (1926).
A. R. Katritzky and C. W. Rees (eds.), Comprehensive Heterocyclic Chemistry, Vols. 1–7, Pergamon, Oxford (1984).
D. O. Holland and J. H. C. Nayler, J. Chem. Soc., No. 6, 1657 (1955).
S. I. Bobrovskii, E. V. Babaev, and Yu. G. Bundel', Khim. Geterotsikl. Soedin_ No. 2, 203 (1987).
J. P. Paolini and R. K. Robins, J. Heterocycl. Chem., No. 2, 53 (1965).
L. Almirante, A. Mugnaini, L. Polo-Fritz, and E. Provincial, Boll. Chim. Farm.,105, 32 (1966).
A. J. Eliot, H. Guzik, and J. R. Soler, J. Heterocycl. Chem., No. 19, 1437 (1982).
F. Mattu and E. Marongiu, Rend. Seminario Fac. Sci. Univ. Cagliari,34, 190 (1964).
F. Mattu and E. Marongiu, Rend. Seminario Fac. Sci. Univ. Cagliari,34, 291 (1964).
F. Mattu, Ann. Chim. (Rome),54, 496 (1964).
C. K. Bradsher, L. D. Quin, and R. E. Leblen, J. Org. Chem.,21, 3273 (1961).
C. K. Bradsher and J. W. McDonald, J. Org. Chem.,27, 4478 (1962).
P. I. Abramenko and V. G. Zhiryakov, Khim. Geterotsikl. Soedin., No. 11, 1544 (1972).
K. Gewald and H. J. Jansch, J. Prakt. Chem.,318, 313 (1966).
G. V. Boyd and N. Singer, J. Chem. Soc. B, 1017 (1966).
K. Waisser, F. Rubacek, R. Kariicek, J. Sova, M. Celednik, and K. Palat, Pharmazie (Berlin),34, 197 (1979).
C. K. Bradsher, R. D. Brandau, J. E. Boliek, and T. L. Hough, J. Org. Chem.,34, 2129 (1969).
C. K. Bradsher and M. F. Zinn, J. Heterocycl. Chem., No. 4, 66 (1967).
Additional information
M. V. Lomonosov Moscow State University, Moscow 119899. Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1104–1111, August, 1995. Original article submitted July 9, 1995.
Rights and permissions
About this article
Cite this article
Babaev, E.V., Efimov, A.V. & Maiboroda, D.A. Hetarenes with a nitrogen bridging atom 1. Phenacylation of 2-substituted 6-methylpyridines. Chem Heterocycl Compd 31, 962–968 (1995). https://doi.org/10.1007/BF01170323
Issue Date:
DOI: https://doi.org/10.1007/BF01170323