Summary
-
1.
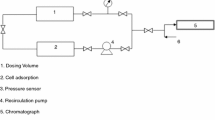
A new volumetric-gravimetric method for the measurement of simultaneous adsorption from binary mixtures of gases or vapors on solid adsorbents is described.
-
2.
Measurements have been made of simultaneous adsorption from mixtures of H2O and C2H5Cl on active charcoal at 74° over a range of relative pressure of water vapor of 0–0.85 and over a range of partial pressures p2 of ethyl chloride of 0–180 mm Hg.
-
3.
The adsorption isotherms for water at constant p2 are displaced by rise in the value of p2 to the right from the origin, and the steepness of their linear middle sections increases.
-
4.
Rise in relative pressureh of water vapor to above 0.5 at constant partial pressure of ethyl chloride leads to a sharp fall in the sorption of ethyl chloride. With further rise inh, the ethyl chloride is practically completely displaced from the charcoal by water.
-
5.
An examination has been made of the stoichiometry of adsorptional displacement in the adsorption of binary gas mixtures, It has been shown. that on the basis of Langmuir's theory, the displacement coefficient γ should tend to unity as the pressure increases, It has been found that in the adsorption of mixtures of H2O and C2H5Cl on charcoal at high pressures γ approximates to the value 5, so that in adsorptional displacement five H2O molecules are equivalent to one C2H5CI molecule.
-
6.
The difficulties arising in the interpretation of the results from the point of view of the concept of capillary condensation of water in the given system are pointed out, and one of the other possible mechanisms of adsorption of water on charcoal is examined: it is based on the hypothesis of two-dimensional change of state in the adsorption layer resulting from the formation of hydrogen bonds between adsorbed water molecules.
Similar content being viewed by others
Literature Cited
B. P. Bering and V. V. Serpinsky, J. Phys. Chem., 26, 253 (1952).
B. P. Bering and V. V. Serpinsky, Proc. Acad. Sci. USSR, 85, 1065 (1952).
B. P. Bering and V. V. Serpinsky, Bull, Acad. Sci. USSR, Div. Chem. Sci., No. 6, 997, (1952).
B. P. Bering and V. V. Serpinsky, Proc. Acad. Sci. USSR, 90, 811 (1953).
B. P. Bering and V. V. Serpinsky, Problems of Kinetics and Catalysis, 7, 383 (1949).
M. M. Dubinin and E. D. Zaverina, J. Phys. Chem., 23, 57 (1949).
N. N. Avgul, O. M. Dzhigit, and A. V. Kiselev, Proc. Acad. Sci., USSR, 86, 95 (1952).
M. M. Dubinin and E. D. Zaverina, Proc. Acad. Sci. USSR, 56, 715 (1947).
M. M. Dubinin and E. D. Zaverina, J. Phys. Chem., 21, 1373 (1947).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bering, B.P., Serpinsky, V.V. Adsorption of gas mixtures. Russ Chem Bull 2, 851–859 (1953). https://doi.org/10.1007/BF01167526
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01167526